Presenting Author:
Rohun Palekar, Ph.D.
Principal Investigator:
Colby Thaxton, M.D.
Department:
Urology
Keywords:
Biosensor, Nanoparticle, Cholesterol, LCAT
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B181 - Basic Science
Nanoparticle-based biosensors for the detection of lecithin:cholesterol acyltransferase (LCAT) activity.
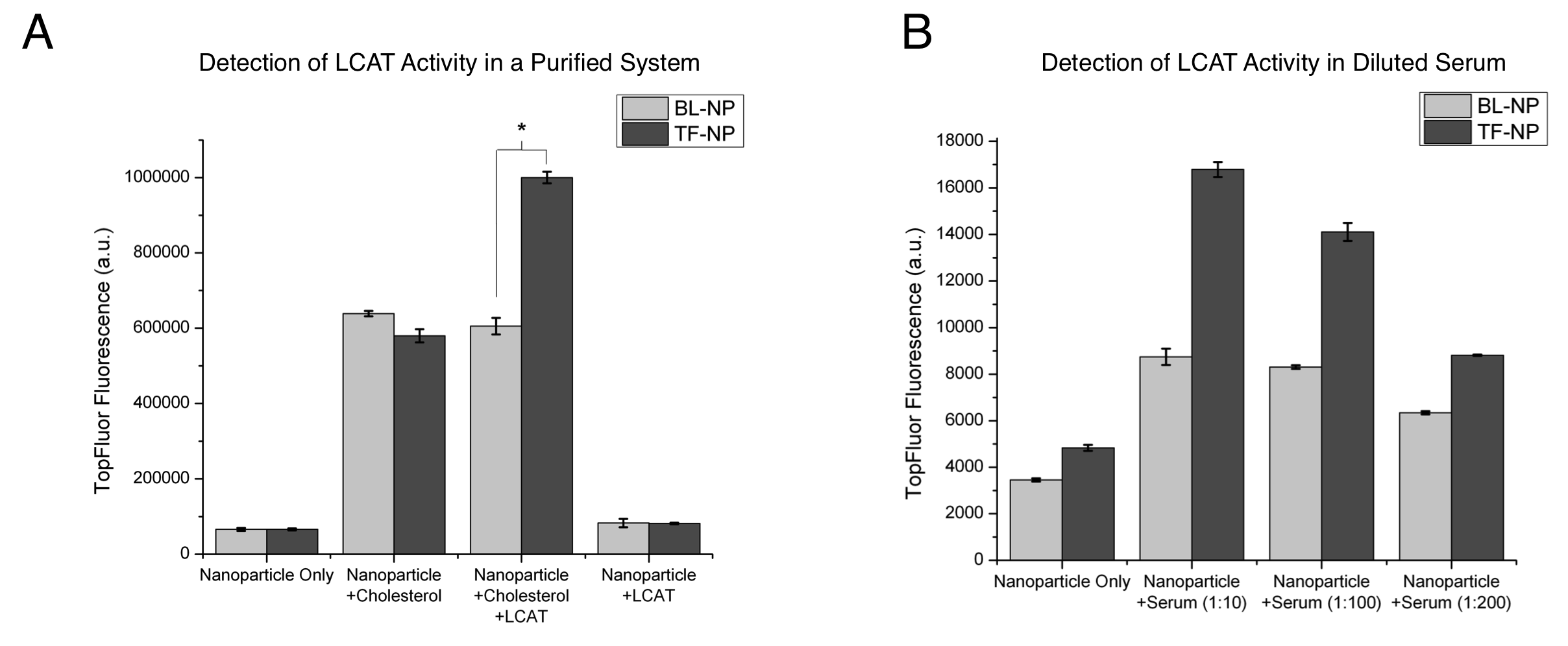
Lecithin:cholesterol acyltransferase (LCAT) is a key enzyme involved in reverse cholesterol transport, a process that may be atheroprotective. Reverse cholesterol transport is the removal of cholesterol from peripheral tissues, transport to the liver, and excretion. Prior research has established that LCAT activity is influenced by external physiological stimuli, and detection of changes in LCAT activity is essential for studying natural atheroprotective mechanisms. Our lab has previously developed nanoparticles (HDL-NP) that mimic the natural functions of HDL, through binding of free cholesterol and serving as substrates for LCAT. Herein, we describe the application of HDL-NP as specific biosensors for assaying LCAT activity. For HDL-NP synthesis, 5 nm diameter gold nanoparticles (Au-NP) were used as a scaffold for the assembly of a lipid layer and ApoAI, a protein found in natural HDL that serves as a cofactor for LCAT. 10% of the total lipid layer consisted of a lipid with a fluorophore (TopFluor) label at the Sn2 position of the lipid, thus forming TopFluor-labeled HDL-NP (TF-NP). A subset of HDL-NP was formulated without ApoAI (BL-NP) to serve as a control. LCAT binding and activity on the NPs was expected to result in an increase in fluorescence due to increased separation of the fluorophore from the quenching effect of the Au-NP core. These nanoparticles were incubated for 24 hours with either cholesterol and LCAT or phosphate buffered saline (PBS), or in a solution of diluted serum to assay the fluorescent response of nanoparticles to cholesterol. TF-NP and BL-NP were incubated with cholesterol alone, LCAT alone, or a cholesterol/LCAT mixture (Fig. A). In this experiment, a significant increase in fluorescent signal was observed for the TF-NP incubated with cholesterol/LCAT over that of the TF-NP alone (p < 0.05). Interestingly, the presence of cholesterol alone with TF-NP and BL-NP results in a fluorescent response, independent of ApoAI content suggesting cholesterol binding displaces the fluorophore from a position in closer proximity to the particle core. TF-NP exhibit significantly increased fluorescence over BL-NP when incubated with cholesterol/LCAT (p < 0.05), demonstrating the importance of ApoAI as an LCAT cofactor. Next, TF-NP and BL-NP were incubated in diluted serum for 24 hours (Fig. B), demonstrating a dose-response increase in fluorescence. Furthermore, this study confirms the specificity of LCAT towards ApoAI-containing TF-NP, where fluorescence intensity was significantly increased over BL-NP groups for all serum dilutions. These results demonstrate the ability of fluorescent HDL-NP to serve as biosensors for detecting LCAT activity, and that serum LCAT activity can be detected using this technology. Future work aims to apply this biosensor for the detection of LCAT activity changes in response to both acute and prolonged exercise, previously demonstrated to induce changes in LCAT activity in human subjects.