Presenting Author:
Hawkins Gay, M.D.
Principal Investigator:
Mark Huffman, M.D.
Department:
Preventive Medicine
Keywords:
Data Sharing, Reproduction Analysis, Strengthening Research, Cardiovascular Trials
Location:
Ryan Family Atrium, Robert H. Lurie Medical Research Center
C109 - Clinical
Cardiovascular Clinical Trial Data Sharing: Reproduction Analysis of SMART-AF Trial
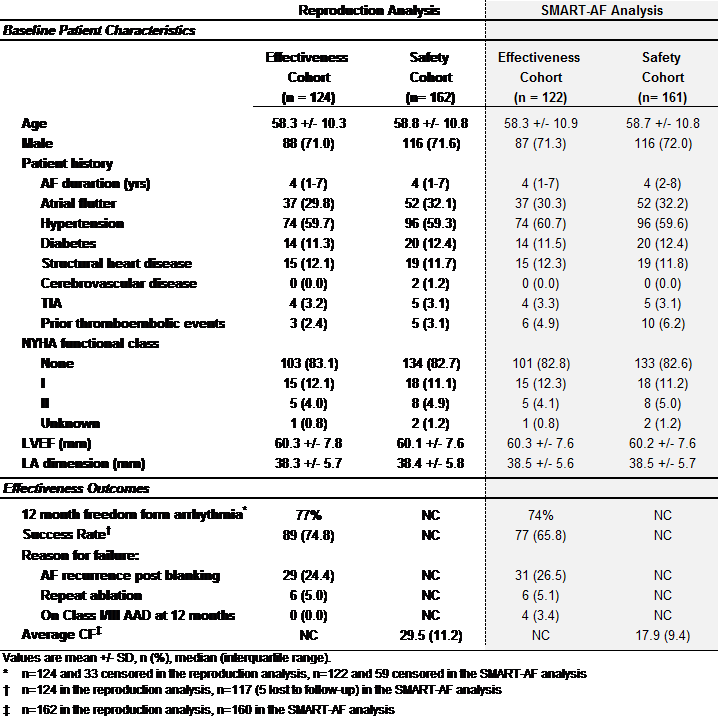
BACKGROUND The National Academy of Medicine has recommended responsible data sharing to maximize benefits from clinical trials. Reproduction analyses are a simple means by which sharing data may strengthen scientific evidence. Yale Open Data Access (YODA) hosts and distributes third-party clinical trial data. We sought access to data from the SMART-AF trial, the only cardiovascular study in YODA, to evaluate the feasibility of a reproduction analysis. METHODS SMART-AF The aim of the multi-center, non-randomized SMART-AF trial was to assess effectiveness and safety of an atrial fibrillation catheter for radiofrequency ablation. The primary effectiveness outcome was the rate of freedom from atrial fibrillation, atrial tachycardia, or atrial flutter through 12 months. The primary safety outcome was the rate of procedure- and device-related serious adverse events. DATA We submitted an application to YODA in May, 2016. Review, revision, submission, and approval of the final data use agreement was completed in October, 2016. Analysis datasets, data dictionary, case report forms and statistical analysis plan were then available through a virtual platform. Statistical code from the original analysis was not included. REPRODUCTION We sought to reproduce the primary effectiveness outcome, as well as tables and figures, from the published SMART-AF trial report. We mirrored, as closely as possible, the original analysis from the methods and statistical plan of the SMART-AF trial. We contacted the study authors, YODA representatives, and trial sponsor for information when reproduction attempts did not match the primary report. Figure 1 shows the full timeline. RESULTS Table 1 compares reproduction and published results. We were unable to exactly match the sample size of the effectiveness (N=124; 122 published) and safety (N=162; 161 published) cohorts described in the primary publication. Baseline demographics by cohort were reproduced with minor differences. We found small differences in the primary outcome of 12-month freedom from arrhythmia recurrence (77% vs 74% published) but larger differences in the calculated success rate (75% vs 66% published; absolute difference = 9.0%, CI: -3.1, 20.9%). The largest difference found was the mean (SD) contact force used during ablation procedures (29.5 g (11.2) vs 17.9 g (9.4) published; absolute difference = 11.6, CI: 9.3, 13.9 g). Further analyses predicated upon incomplete information could not be completed. CONCLUSIONS We demonstrate the feasibility of a reproduction analysis from the SMART AF trial, the first cardiovascular trial posted to YODA, albeit with some differences that may be clinically relevant. The trial authors and sponsor have exhibited leadership in data sharing in cardiovascular research. Future reproduction could be completed with higher fidelity by sharing statistical code, or quicker with more streamlined, centralized processes and platforms, such as NIH BioLINCC.