Presenting Author:
Principal Investigator:
Andrew Larson, Ph.D.
Department:
Radiology
Keywords:
bacteriolytic therapy, clostridium novyi-NT, magnetic resonance imaging, MRI, iron oxide labeling, hepatocellular carcin... [Read full text]
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B172 - Basic Science
Iron Oxide Labeled Clostridium novyi-NT Spores for Image-Guided Bacteriolytic Therapy
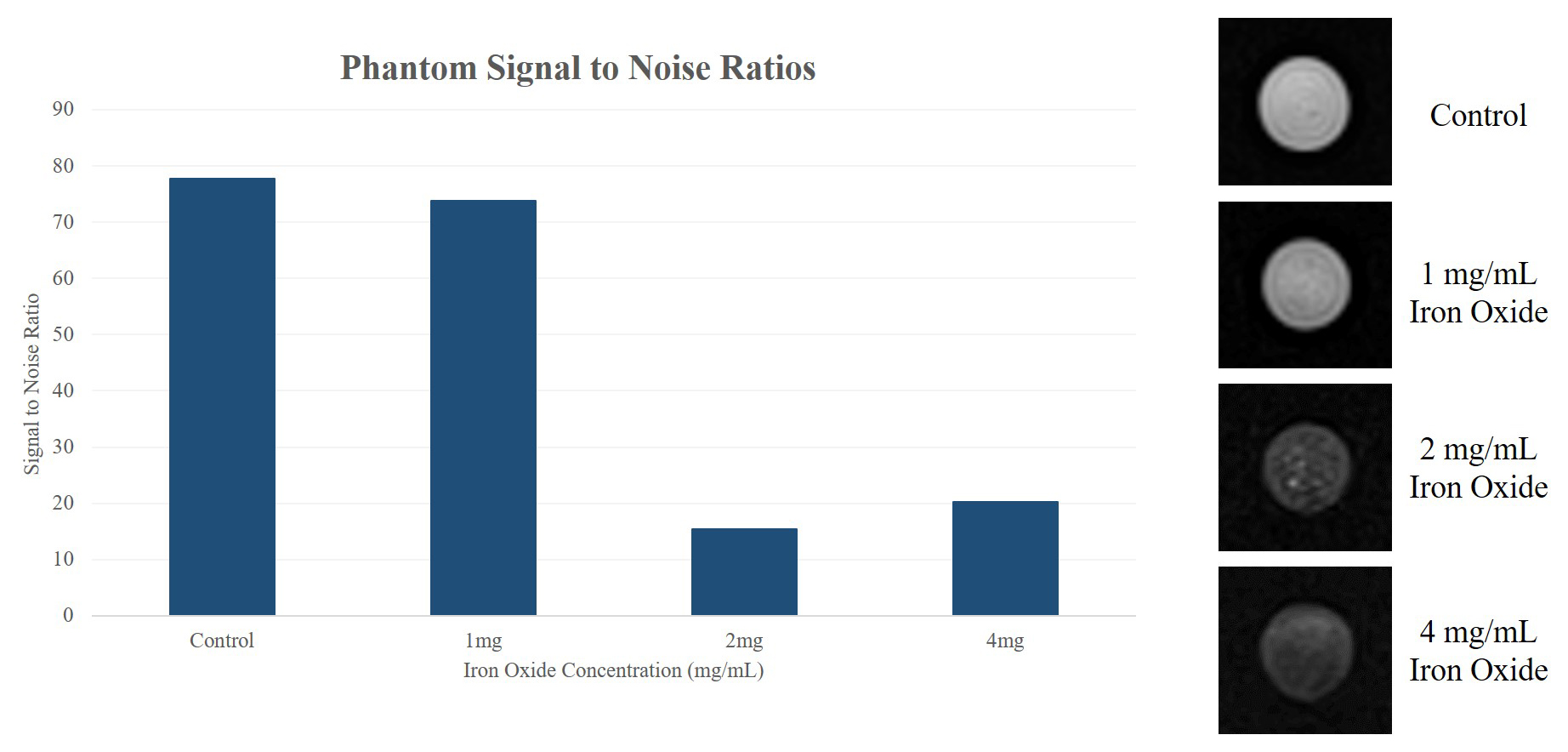
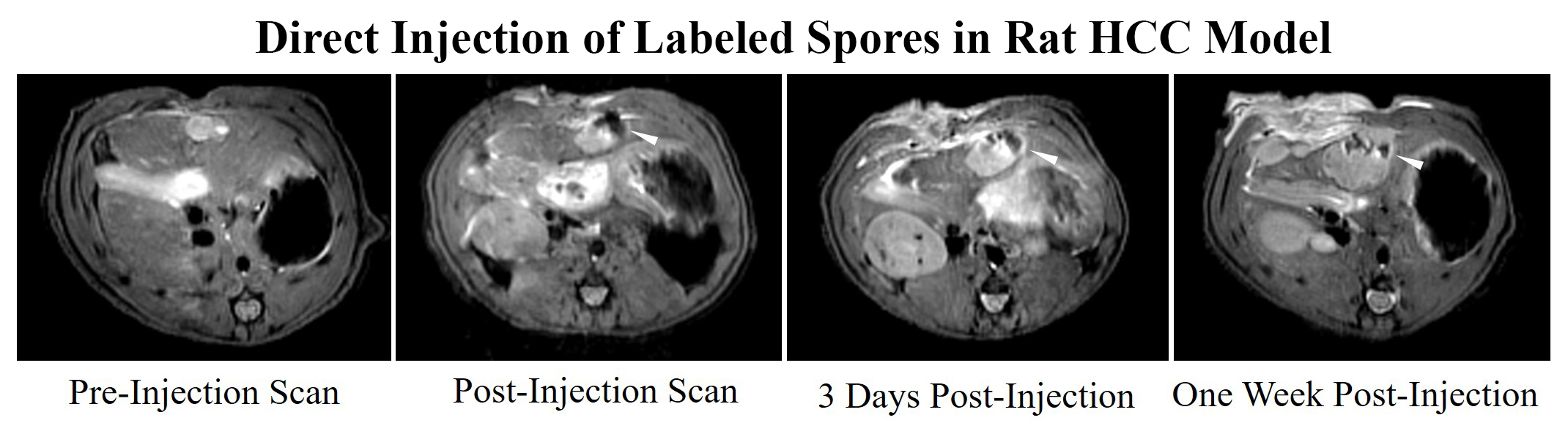
Background: Clostridium novyi-NT is an obligate anaerobe commonly used in bacteriolytic therapy. This modified bacteria can specifically treat necrotic regions of tumors without harming the rest of the organism. However, there is no current method to confirm delivery of dormant bacterial spores and the main method of delivery to date is to systemically infuse spores into the bloodstream in hopes a small subset will reach and colonize the tumor. Given the concern of toxicity from systemic infusion, a safer method as well as the ability to confirm delivery is still necessary before bacteriolytic therapy can be applied widely in the clinical environment. Purpose To develop iron-oxide labeled Clostridium novyi-NT spores for MRI visualization during bacteriolytic therapy and to demonstrate that MRI visualization of spore delivery can be valuable for intra-procedural optimization of tumor-directed administration procedures and early prediction of treatment outcomes. Materials and Methods: Iron Oxide Labeling: Vegetative state C. novyi NT were incubated with iron oxide particles coupled with rhodamine B (Ocean Nanotech, Inc.) at a concentration of 100µg/mL. Resulting bacteria underwent viability studies comparing germination rates as measured by optical density. Confocal microscopy was used to evaluate labeling efficacy and TEM imaging was performed to confirm the presence of iron within the spores. Phantom Studies: MRI phantoms of spores incubated with various concentrations of iron oxide were constructed by homogeneously mixing 4 x 10^8 labeled spores with 250µL of 2% agarose. T2*-weighted images of the phantoms were acquired using a 7T MR scanner. In vivo Studies: In Sprague Dawley rats, N1-S1 liver tumors were directly injected with bacteria and imaged immediately, three days, and one week after injection. In a VX2 liver tumor rabbit model, bacterial spores were infused using a catheter guided approach through the hepatic artery and animals were imaged immediately before and after treatment on MRI. Results: Labeled spores demonstrated similar germination rates as control spores. Fluorescence microscopy demonstrated labeling efficacy of 100% and TEM showed diffuse deposition of iron oxide within the spores. T2*-weighted imaging showed significant signal decay in the spores labeled with 2mg/mL and 4 mg/mL of iron oxide compared to controls. In rats, labeled spores could be visualized with MRI immediately, 3 days, and one week after direct injection into tumors. Spores were also visible on MRI surrounding the liver tumor in the rabbit model immediately post-infusion. Conclusion: C. novyi NT spores can be efficiently labeled with iron oxides by inducing sporulation in labeled vegetative state bacteria. These methods have direct applications for image-guided bacteriolytic therapy and are permitting ongoing comparisons between IV and direct injection administration routes as well as the investigation of intra-arterial catheter-directed alternatives.