Presenting Author:
Ann Carias, Ph.D.
Principal Investigator:
Thomas Hope, Ph.D.
Department:
Cell and Molecular Biology
Keywords:
antibodies, HIV, vaginal epithelium, non-human primate model
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B15 - Basic Science Women's Health Research
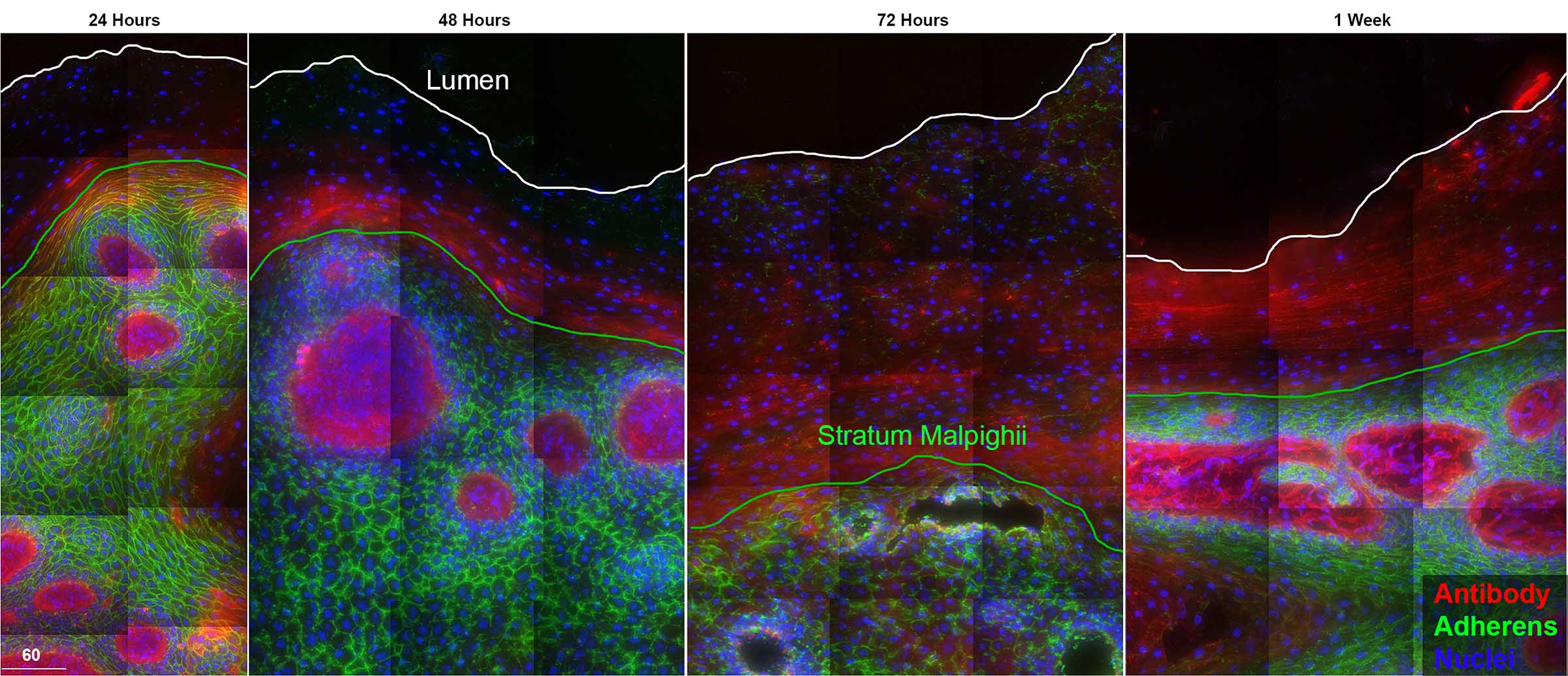
Distribution and localization of fluorescently-tagged transferred antibodies in the vagina
In the world of host-HIV interactions, non-neutralizing and broadly neutralizing antibodies have been illustrated to provide protective responses against lentiviral infections. Antibody passive transfer studies in non-human primates have demonstrated protection against HIV infection; however, the mechanism of this protection at mucosal sites is unknown. Through various approaches, we have determined antibody half-life, along with where these antibodies localize and how they enter into vaginal mucosal tissues. To define antibody dynamics and localization in vivo, rhesus macaques were intravenously administered fluorescently tagged non-specific Gamunex-C, HIV-specific VRC01, VRC01-LS or a combination of two of aforementioned antibodies. Additional macaques were also injected with fluorescently tagged Gamunex-C and human serum albumin. Following injection, serum, vaginal wecks, sequential vaginal biopsies, and whole necropsied vaginal tissues were collected at time points ranging from 24hours to 8 weeks. With each sample, antibody levels in serum and wecks were analyzed using fluorometric spectroscopy. Tissue fluorescent levels were measured with deconvolution microscopy. Antibodies were detectable in sera, wecks, and tissue as early as 24 hours and up to ~4 weeks. In all samples, antibodies reached a steady state between 1-2 weeks, however, Gamunex-C persisted longer in sera than VRC01 or VRC01-LS. Antibodies, both non-specific and HIV-specific, were found to first appear in the vaginal lamina propria in an unidentified subset of epithelial cells and moved into the superficial layers through epithelial cell differentiation. Likewise, human serum albumin moved through the vaginal epithelium in a similar fashion. Here, we illustrate how antibodies can passively enter mucosal tissues after intravenous administration revealing endogenous pathways of antibody distribution in mucosal squamous epithelium. Within the vaginal epithelium, we observe an apparent cell-based mechanism of antibody delivery where a subset of basal epithelial cells capture antibodies from tissue fluids and then migrate towards the surface, eventually delivering antibody to the lumen. The migration of serum albumin and antibody were synchronized suggesting we are observing the kinetics and mechanism of transudation. These data prove essential for understanding and developing novel antibody-based preventions of HIV infection.