Presenting Author:
Arangassery Rosemary Bastian, Ph.D.
Principal Investigator:
Thomas Hope, Ph.D.
Department:
Cell and Molecular Biology
Keywords:
HIV, female reproductive tract, cervical mucin, MUC5AC, antibody, immunoglobulin, mucosal vaccine, antibody neutralizati... [Read full text]
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B14 - Basic Science Women's Health Research
Multivalent Immunoglobulin Muc5AC Complexes Enhance Antibody Potency Against HIV-1
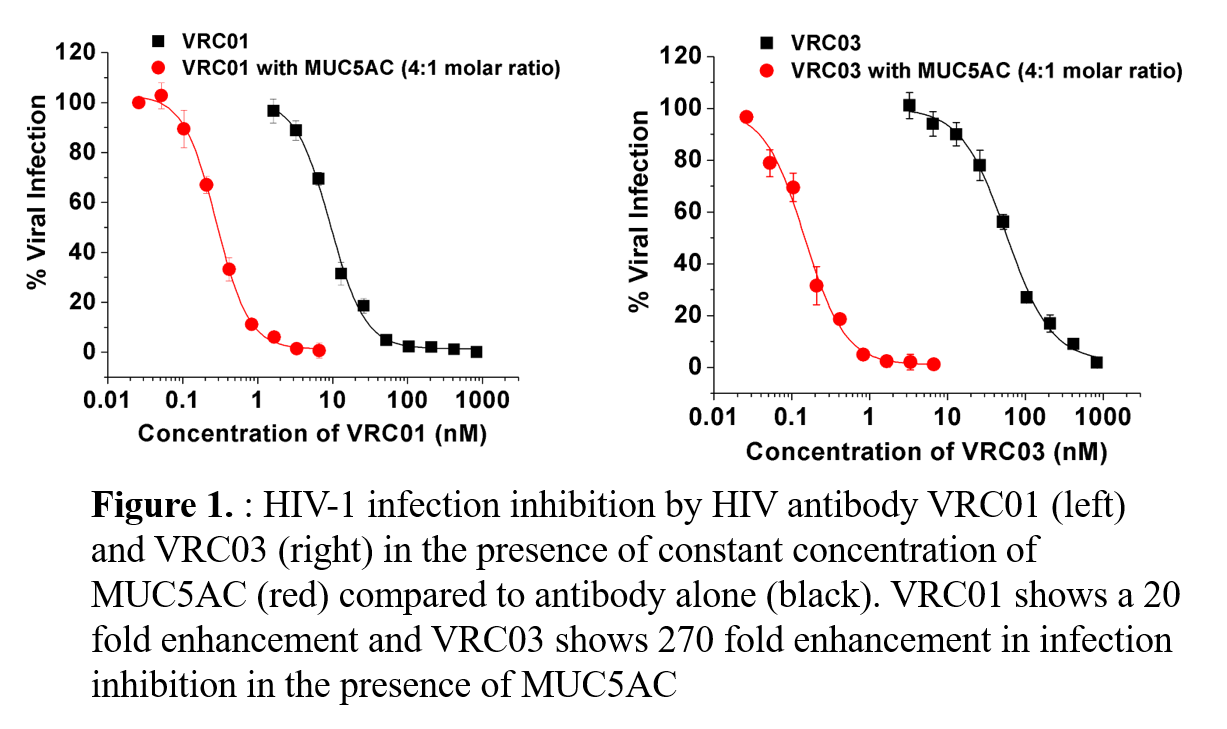
Mucus is made up of mucins and other secreted proteins including immunoglobulins (IgGs). Mucus acts as a natural barrier system of the female reproductive tract (FRT) against pathogen invasion. A critical goal in HIV prevention is the development of a mucosal vaccine that would provide long-term protection at the site of virus acquisition including the FRT. Previous studies have shown that cervical mucus leads to reduction in mobility of pathogens; however our understanding of the function of IgGs within complex mucosal secretions and how this aids the mucosal barrier is limited. We recently found that multiple IgG Fc can bind to mucins in the FRT with nanomolar affinity. This study seeks to quantify the binding of IgGs to mucin and the ability of the complexes to increase the activity of Env directed IgG against HIV infection. To study the mucin-IgG interaction we utilized MUC5AC, a gel-forming mucin subtype, and the abundant mucin secreted in the cervix of the human FRT. The stoichiometry, thermodynamics, and kinetics of the mucin-IgG interaction was studied using isothermal calorimetry and surface plasmon resonance. The in vitro neutralization potency of a variety of neutralizing and non-neutralizing anti-HIV IgGs antibodies was determined in the presence and absence of MUC5AC. Single virus particle tracking and rheology were used to visualize and quantify the interaction of HIV with these mucin-IgG complexes. Our results show that MUC5AC-IgG has nanomolar binding affinity (KD ~60nM), with approximately 8 IgGs binding to MUC5AC, resulting in a multivalent mucin-IgG complex that can interact with HIV. The potency of in vitro neutralization with certain anti-HIV IgGs, VRC01, VRC03, VRC06 and polyclonal HIVIg can synergistically increase several folds when complexed with MUC5AC. In other cases, non-neutralizing anti-HIV IgGs can be converted to have potent neutralizing activity when complexed with MUC5AC. We also observe that MUC5AC-IgG complexes can hinder HIV diffusion when complexed and further lead to alteration in mucin-mucin interactions. We demonstrate that MUC5AC forms a multivalent, high affinity immune complex with IgG, thereby revealing a new effector function of antibodies at the mucosal surface. We show that MUC5AC leads to an increase in the antiviral activity of most HIV antibodies and induces anti-viral activity in non-neutralizing HIV antibodies. Our results indicate that MUC5AC-IgG-HIV interactions not only enhance anti-viral potency but can also alter mucin-mucin interactions. Our results suggest that there an important role in the interplay between innate and adaptive immune systems to form a more effective anti-pathogen barrier. These findings will enhance the understanding of antibody function at mucosal surfaces and provide insight into mucosal vaccine mechanisms against pathogens like HIV, influenza and HPV.