Presenting Author:
Renea Jablonski, M.D.
Principal Investigator:
Renea Jablonski, M.D.
Department:
Medicine
Keywords:
Eosinophil, macrophage, lung fibrosis
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B74 - Basic Science
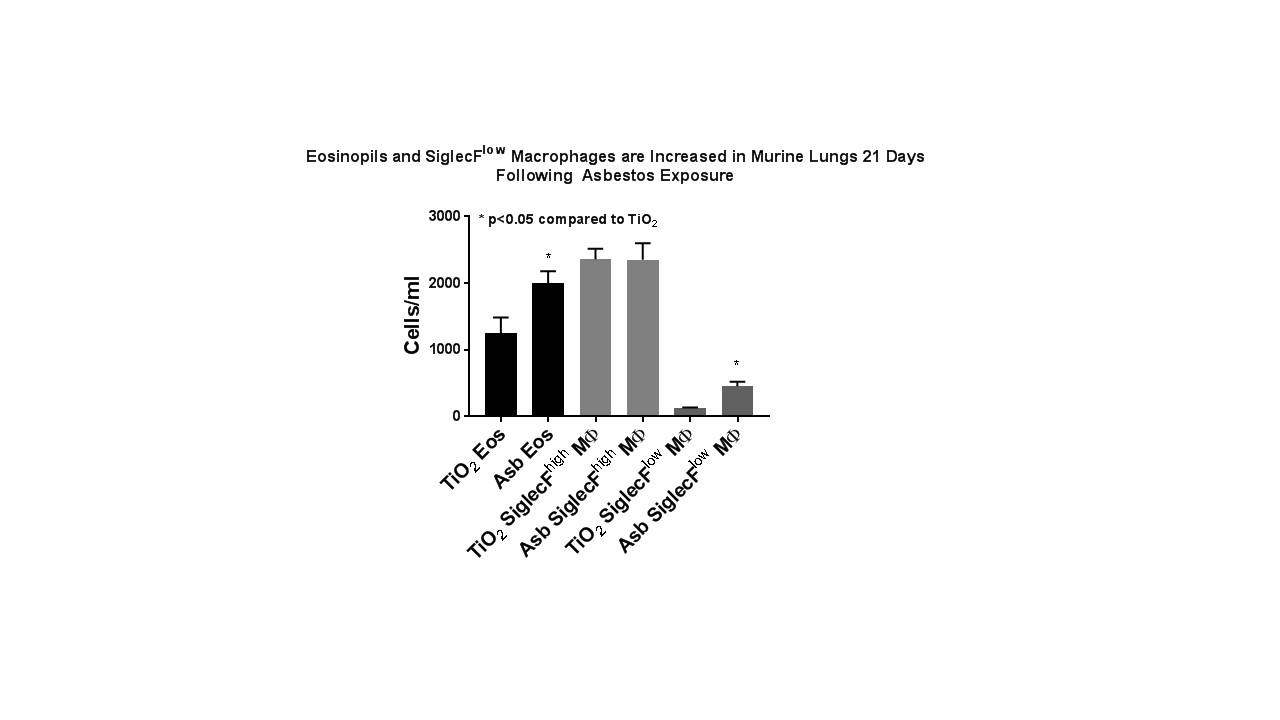
Eosinophils and monocyte-derived macrophages are increased in asbestos-induced lung fibrosis
Rationale: Alveolar epithelial cell (AEC) injury plays a vital role in the development of IPF and asbestosis. We showed that asbestos-induced lung fibrosis in mice is due in part to type 2 AEC cell mitochondrial DNA (mtDNA) damage and intrinsic apoptosis (Cheresh et al AJRCMB 2015). Although inflammatory cells are present in lung fibrosis, anti-inflammatory therapy has been largely unhelpful in the treatment of IPF. Our group has been using flow cytometry to allow for unambiguous identification of myeloid populations in the murine and human lung, which provides information independent of BAL (Misharin et al AJRCMB 2013, Bharat et al AJRCMB 2016). Targeted anti-eosinophil therapy using anti-IL-5 antibodies attenuates bleomycin-induced lung fibrosis (Gharee-Karin et al JLB 1998) but the role of eosinophils in modulating asbestos-induced lung fibrosis and the detailed molecular mechanisms involved are unclear. Methods: Male and female 8-10 week-old WT mice were exposed to a single intratracheal instillation of crocidolite asbestos (100 µg/100 µL) or TiO2 (100 µg/100 µL, negative control). The lungs were harvested 21 days later and, following CD45 enrichment, cells were stained, fixed and subject to flow cytometric analysis as previously described (Misharin et al AJRCMB 2013). Results: Compared to controls, mice exposed to asbestos had a significant increase in lung eosinophils (see Figure, 1.6-fold compared to control), specifically due to the emergence of a recruited SiglecFhighCD11c+ population, which was recently described in asthma models (Valencia et al Allergy 2016). We also see the emergence of a population of SiglecFlow, monocyte-derived alveolar macrophages (3.7-fold increase compared to control) which have previously been associated with bleomycin-induced fibrosis. Conclusions: Our findings demonstrating expansion of recruited lung eosinophils and monocyte-derived alveolar macrophages in asbestos-induced lung fibrosis suggest that strategies that better characterize lung inflammatory cells at the site of asbestos-induced AEC injury may mitigate lung fibrosis. Additional data describing epithelial-inflammatory cell cross talk may help prevent ongoing inflammatory cell recruitment and injury leading to fibrosis. The novel finding of increased lung eosinophils suggests that anti-eosinophil therapies currently on the market may prove to be rational, innovative targets for IPF which are sorely needed as existing therapies do not stop or reverse declines in lung function. Further experiments to characterize the time course of inflammatory cell recruitment and eosinophil-deficient mice are protected from asbestos-induced lung fibrosis, AEC mtDNA damage and apoptosis are ongoing in our lab.