Presenting Author:
Carrie Yuan, B.S.
Principal Investigator:
Catherine Hunter, M.D.
Department:
Pediatrics
Keywords:
Necrotizing enterocolitis, NEC, Caco-2, Rock Inhibitor Y-27632, claudin-2, permeability, lipopolysaccharide
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B146 - Basic Science
Inhibition of Rho-Kinase Decreases Intestinal Permeability in Experimental Necrotizing Enterocolitis
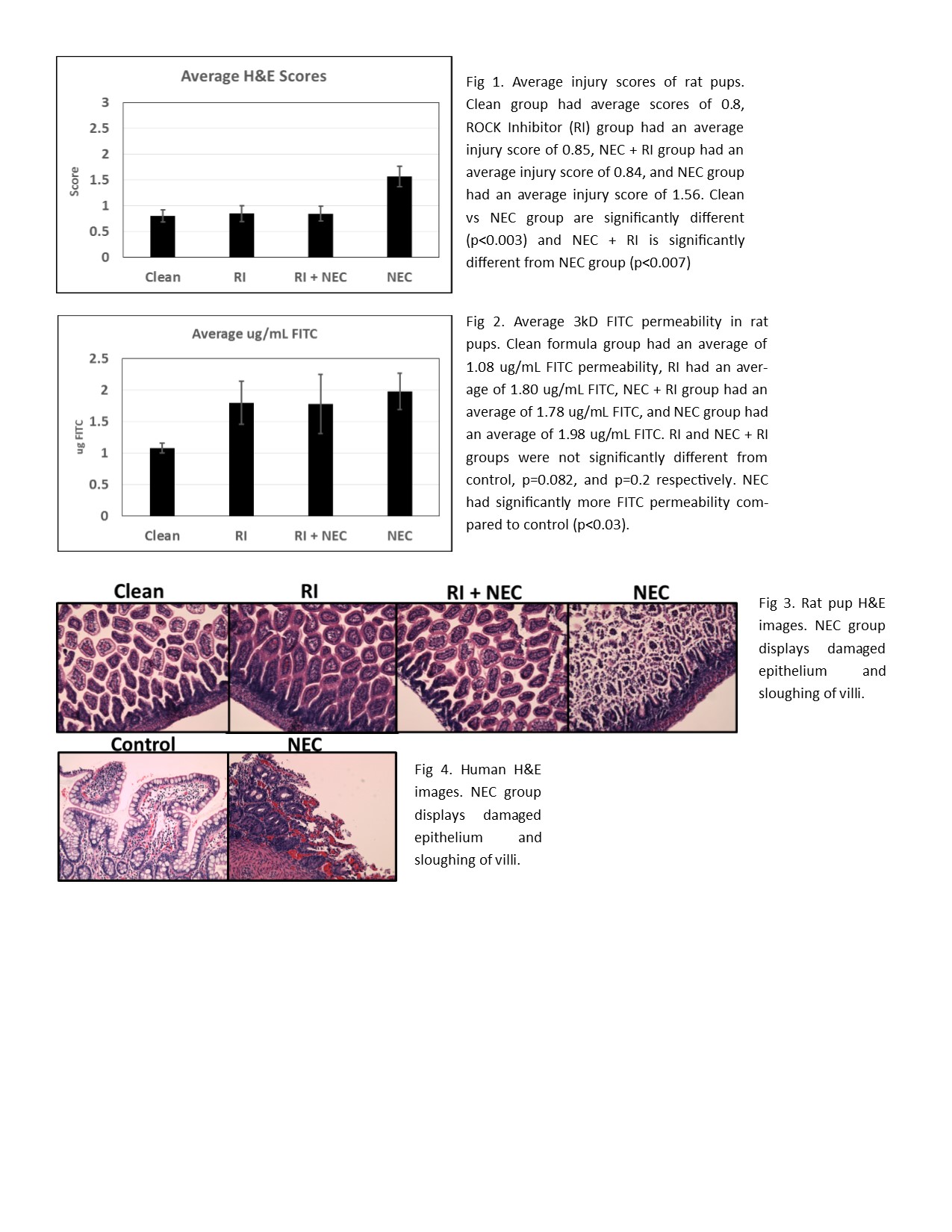
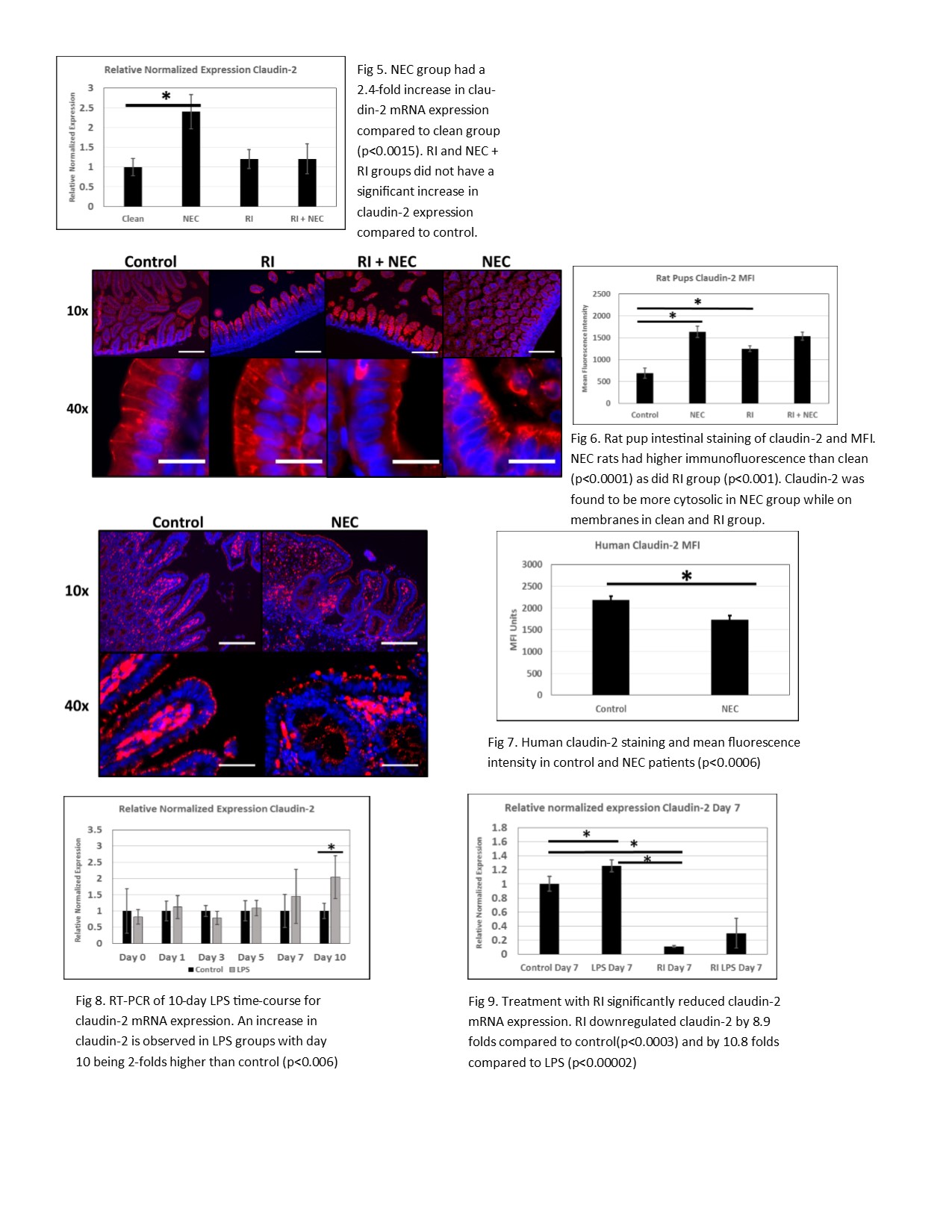
Introduction: Necrotizing enterocolitis (NEC) is a devastating neonatal gastrointestinal disease. NEC makes up approximately 5% of all neonatal intensive care unit admissions. Despite years of investigation, the pathophysiology remains unknown. Tight junctions (TJ) are protein complexes in apex of intestinal villi and attach to the perijunctional actomyosin ring. Disruptions in TJ have been observed in NEC. Rho-Kinase (ROCK) is implicated in several cellular processes including TJ dysfunction. ROCK leads to phosphorylation of myosin light chain (MLC) leading to contraction of the actomyosin ring. Claudin-2, a pore-forming TJ protein, is upregulated in intestinal diseases and increases permeability. We hypothesize that an interaction exists between MLC-induced contractility and claudin-2 and that in a rodent and cell model of NEC the inhibition of ROCK will downregulate claudin-2 and decrease permeability. Methods: In vivo: Rat pups were subjected to hypoxia twice a day and fed either clean formula (control), bacteria + formula (NEC), ROCK inhibitor Y-27632 (RI), and ROCK inhibitor + bacteria (RI + NEC). Gavage fed FITC-dextran was used to assess membrane permeability. H&E slides of intestinal segments were scored by a pathologist blinded to the groups for assessment of disease severity. Claudin-2 was imaged by immunofluorescence and mean fluorescent intensity measured (MFI). In vitro: Caco-2 cells were grown on a transwell system with induction of NEC using lipopolysaccharide (LPS). Transepithelial resistance (TEER) of Caco-2 were measured. Claudin-2 expression levels were analyzed in both rat and cells by RT-PCR. Statistical analysis was performed by ANOVA and student’s T-test. Results: H&E slides revealed a significant increase in injury scores in the NEC pups as compared to the control (p<0.003). RI + NEC group had a significant decrease in injury score compared to the NEC group (p<0.007) and no difference compared to the control group. FITC-dextran permeability was significantly increased in the NEC pups (p<0.03). RT-PCR analysis showed that NEC pups had a 2.4 fold increase in claudin-2 expression compared to control (p<0.015) while RI group had no difference. Claudin-2 immunofluorescence showed an increased MFI in NEC pups. Addition of LPS to Caco-2 cells had a TEER decrease of 15% by day 10 compared to untreated cells. Claudin-2 expression in Caco-2 was increased in LPS groups by day 7 by at least 1.2 folds (p<0.04). Treatment with ROCK inhibitor decreased claudin-2 expression by 8.9 folds compared to control (p<0.003) and ROCK inhibitor + LPS was decreased by 3.34 folds. Conclusion: In conclusion, both rodent and Caco-2 cell models of NEC has increased claudin-2 expression and increased permeability. Inhibition of ROCK decreased claudin-2 expression in both rats and Caco-2 models. These data suggests that ROCK inhibitors could be valuable in the treatment of NEC.