Presenting Author:
Pieter Norden, Ph.D.
Principal Investigator:
Tsutomu Kume, Ph.D.
Department:
Medicine
Keywords:
transcriptional regulation, angiogenesis, lymphatic development, cellular signaling pathways, murine models of development
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B56 - Basic Science
Transcriptional regulation of postnatal vascular development by Foxc1 and Foxc2
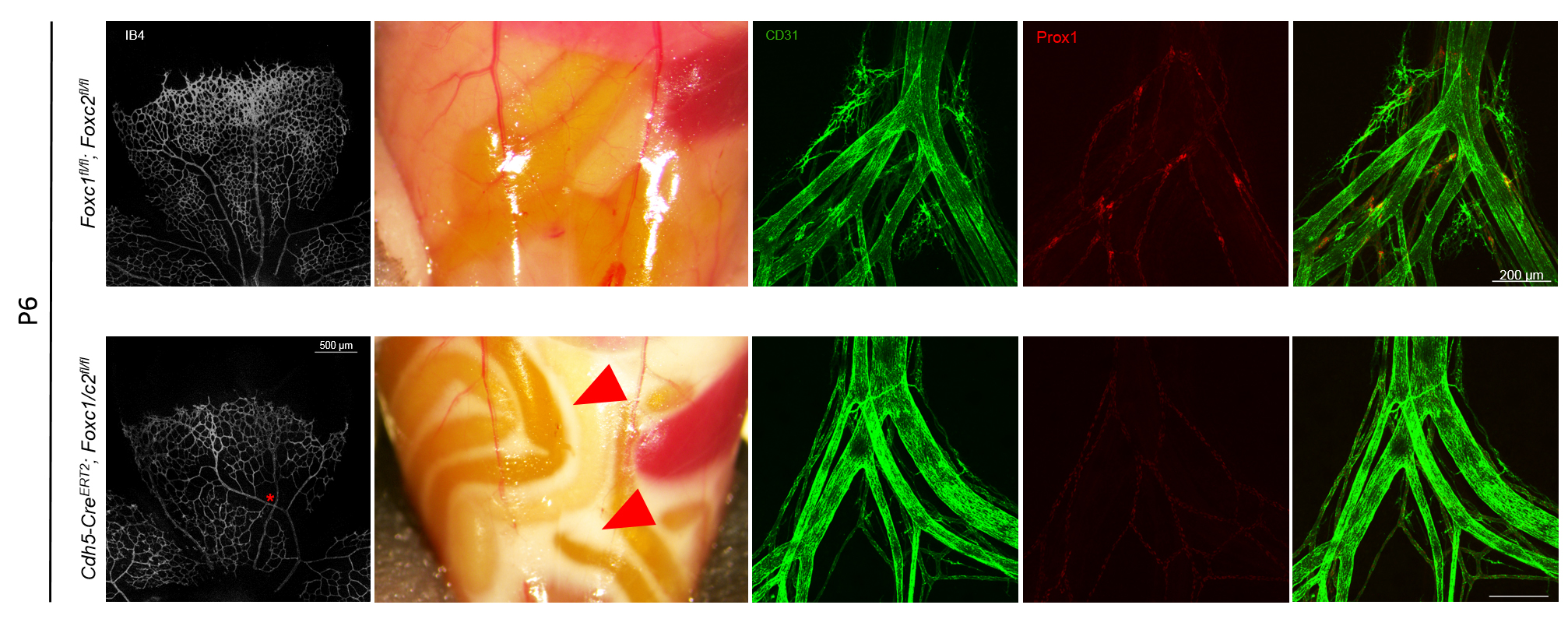
Mutations in the forkhead box (FOX) transcription factors FOXC1 and FOXC2 are associated with Axenfeld-Rieger syndrome, characterized by congenital ocular defects and ventricular septal defects, and late-onset lymphedema respectively. During murine embryonic development Foxc1 and Foxc2 show overlapping expression in many embryonic tissues derived from the non-axial mesoderm and global or endothelial-specific excision of Foxc1, Foxc2 or both results in a number of vascular abnormalities. It has been previously demonstrated that these abnormalities are related to arterial-venous specification via interaction with the VEGF-Notch signaling pathway, ERK activity and proper vessel morphogenesis during lymphangiogenesis, and lymphatic valve formation, maturation and maintenance, often resulting in embryonic lethality. Because the role of these transcription factors in postnatal vascular development is not well understood, we have generated transgenic mice containing tamoxifen inducible VE-Cadherin Cre recombinase and Foxc1fl/fl or compound Foxc1fl/fl; Foxc2fl/fl sites to investigate mechanisms of angiogenesis in the murine retina, which undergoes rapid vascular growth followed by remodeling from approximately age P1 to P21, and maturation and maintenance of mesenteric lymphatic collecting vessel valves that form from E15-16 to P1 and continue to mature. Here, we demonstrate that early postnatal excision of Foxc1 or both Foxc1 and Foxc2 by administration of Tamoxifen from P1-P5 impairs vessel density, branching index and retinal expansion in P6 individuals. Additionally, we identify a critical role for Foxc1 and Foxc2 in lymphatic valve maintenance and maturation where combined deletion during early postnatal development results in near ablation of lymphatic valves in the mesentery and individuals present chylous ascites. Continued investigation into mechanisms regulated by these transcription factors related to vascular patterning, endothelial metabolism, survival, and cytoskeletal organization is of great importance and is critical for understanding cardiovascular and lymphatic pathologies.