Presenting Author:
Gurmit Singh, B.S.
Principal Investigator:
Erin Hsu, Ph.D.
Department:
Orthopaedic Surgery
Keywords:
Trisulfated GPA, rhBMP, Peptide Amphiphile.
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B122 - Basic Science
A Trisulfated Glycopeptide Amphiphile Nanofiber Scaffold for Spinal Arthrodesis
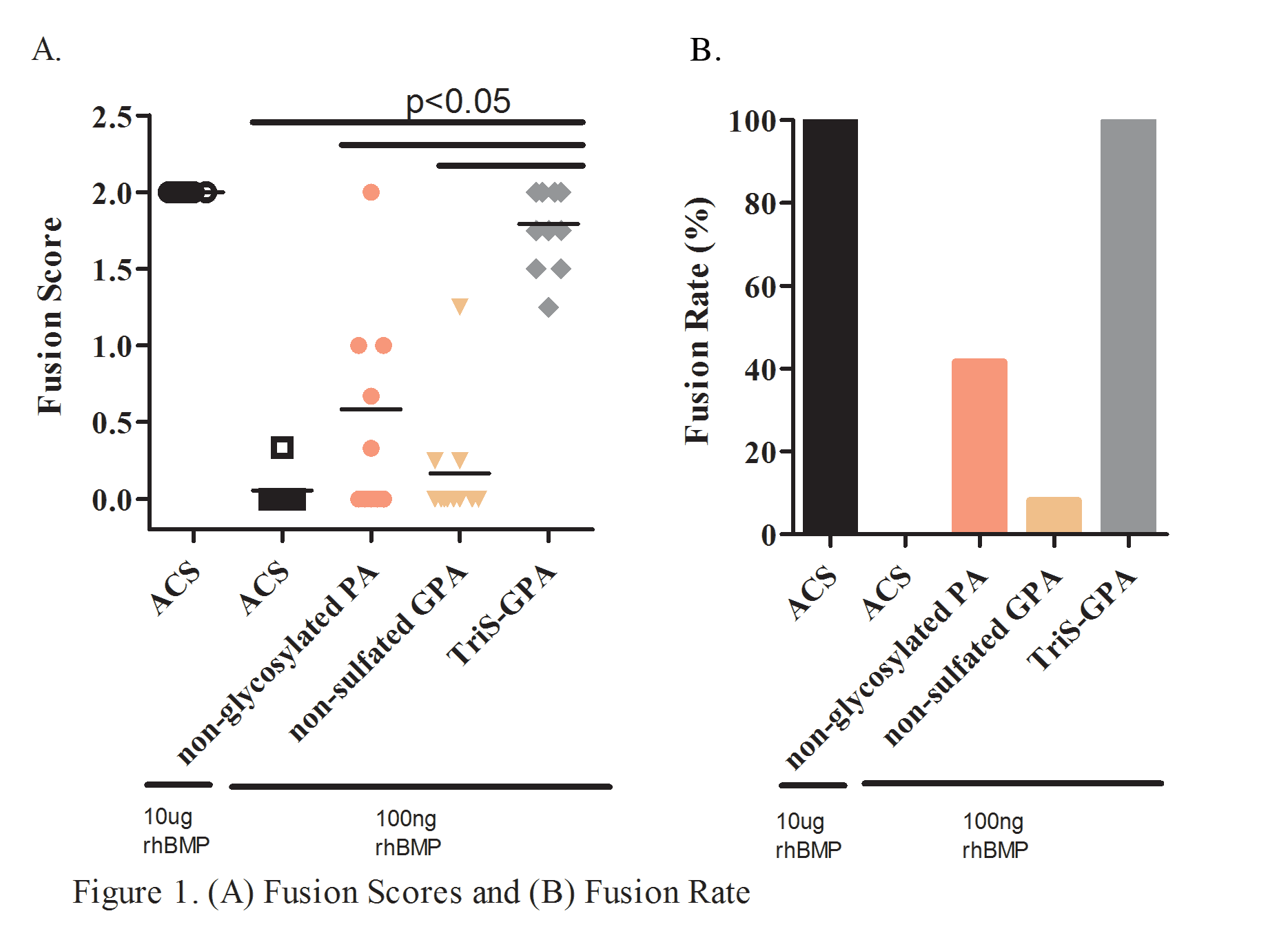
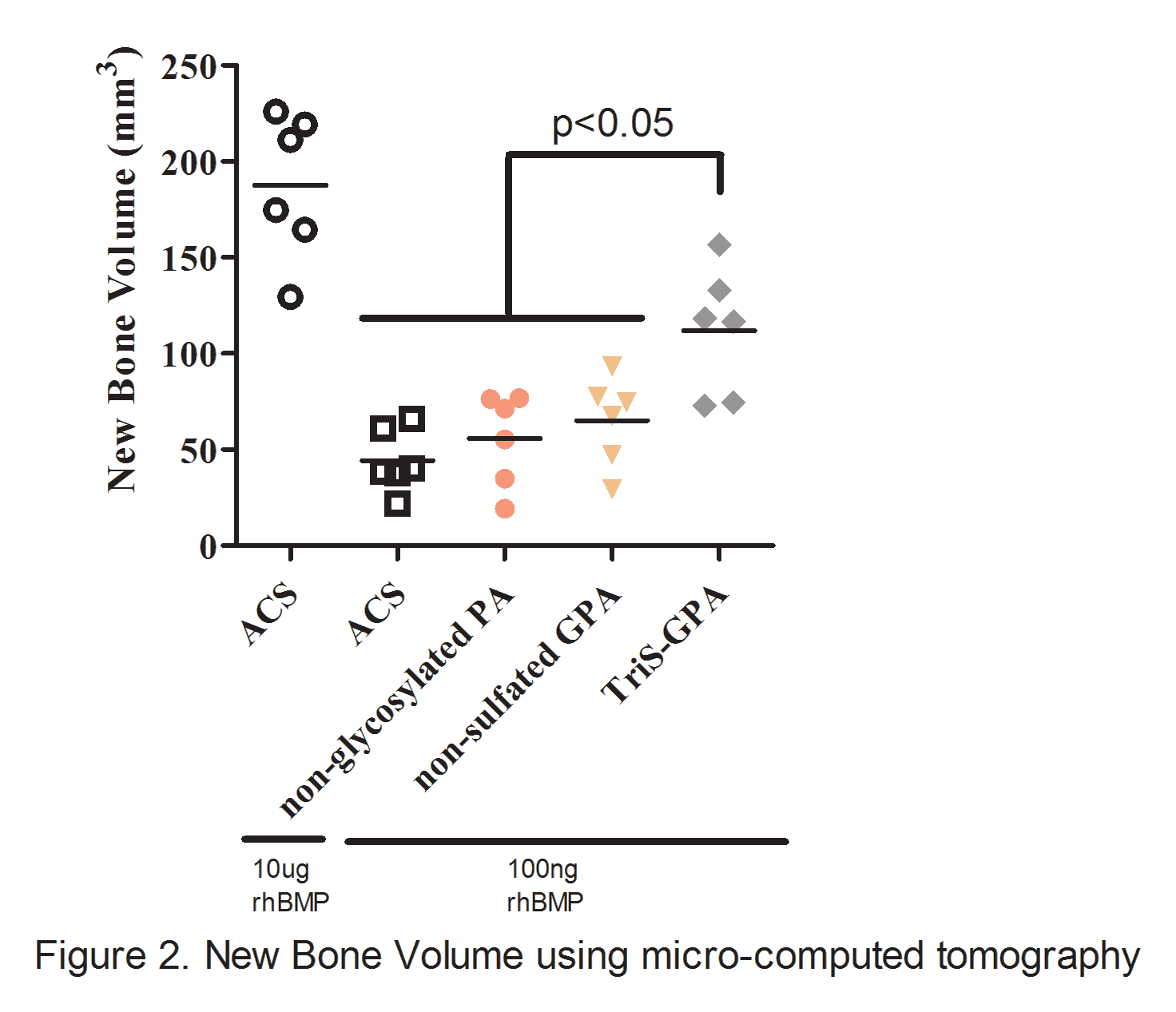
INTRODUCTION: Use of recombinant human BMP-2 (rhBMP-2) has decreased the rate of pseudarthrosis after spine fusion procedures. However, reported complications have provoked considerable interest in the development of novel scaffolds, such as self-assembling nanofiber peptide amphiphiles (PA), that allow for reduced concentrations of rhBMP-2 without compromising its therapeutic effects. Our lab has shown that PAs synthesized with a trisulfated monosaccharide (3,4,6S-GlcNAc PA or TriS-GPA) mimic natural glycosaminoglycans (GAGs) such as heparin and heparan sulfate and can bind to BMP-2. The aim of this study was to evaluate the efficacy of a trisulfated-GPA nanofiber scaffold in promoting spinal arthrodesis in a rat posterolateral fusion (PLF) model. We hypothesized that the TriS-GPA scaffold would increase lumbar fusion rates relative to non-sulfated GPA, non-glycosylated PA and ACS scaffold with equivalently-loaded rhBMP-2. METHODS: Female Sprague-Dawley rats underwent L4-L5 PLF procedure with placement of one of four absorbable collagen sponge (ACS) scaffolds preloaded with 100 ng of rhBMP: (1) ACS + TriS-GPA, (2) ACS + GlcNAcPA (non-sulfated GPA), (3) ACS + PA (non-glycosylated PA) or (4) ACS alone (negative control). An animal group treated with ACS scaffolds pre-loaded with 10 μg rhBMP-2 (per animal) was used as a positive control. Eight weeks post treatment, spines were evaluated for successful fusion and new bone formation via radiographs, manual palpation, microCT imaging, and histologic analysis. Fusion scores were determined by blinded palpation using an established scoring system: 0 = no bridging bone, 1 = unilateral bridging, and 2 = bilateral bridging bone. Spines with an average score of ≥ 1.0 were considered successfully fused. RESULTS: TriS-GPA preloaded with 100 ng rhBMP-2 elicited significantly higher fusion scores relative to ACS, control (non-sulfated) GPA, and non-glycosylated PA (p<0.001; Figure 1A). Animals treated with TriS-GPA elicited a fusion rate of 100%, which was also significantly higher than animals treated with ACS, non-glycosylated PA, and non-sulfated GPA (0%, 42%, 8%, respectively; Figure 1B) with equivalently preloaded rhBMP-2. MicroCT analysis highlighted significantly higher bone volume in the group treated with TriS-GPA compared to all other groups except the positive control (Figure 2). DISCUSSION: Our results suggest that PAs synthesized with a trisulfated monosaccharide (TriS-GPA) can effectively serve to bind and sequester BMP-2 at the site of bone formation. TriS-GPA on an ACS carrier was capable of eliciting lumbar spine fusion while utilizing a lower dose of rhBMP-2 than the established 100% fusion positive control model. SIGNIFICANCE: Reducing the amount of rhBMP-2 necessary for successful spine fusion through the development of novel carriers could decrease the rate of side effects observed with supraphysiologic dosing of rhBMP-2 currently used.