Presenting Author:
Principal Investigator:
Tamara Isakova, M.D.
Department:
Medicine
Keywords:
Randomized controlled trial, fibroblast growth factor 23, phosphate, chronic kidney disease, nicotinamide, cholestatic l... [Read full text]
Location:
Ryan Family Atrium, Robert H. Lurie Medical Research Center
C47 - Clinical
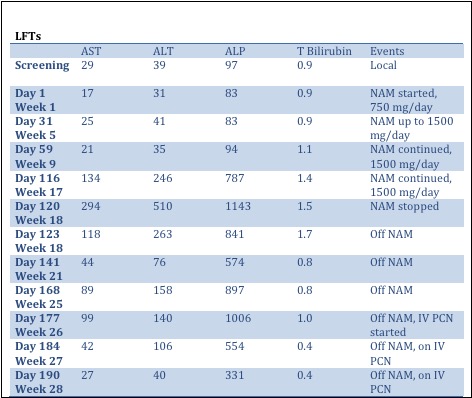
Cholestatic liver injury in a study participant
Background The COMBINE study is a randomized, double-blinded, placebo-controlled, parallel group, 12-month study examining the effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor 23 (FGF23) levels in 205 patients with stage 3-4 chronic kidney disease (CKD). Here, we report a case of a 45-year-old man with type 1 diabetes and stage 4 CKD (estimated glomerular filtration rate [eGFR] of 27 ml/min/1.73m2 at study entry), who developed cholestatic liver injury during the study. Methods We conducted a literature search on potential causes for cholestatic liver injury and focused on abnormal liver function tests (LFTs) in patients taking nicotinamide (NAM), in addition to reviewing diagnostic data from our patient. Results The patient’s past medical history was significant for type 1 diabetes complicated by stage 4 CKD, retinopathy and coronary artery disease. The patient’s early course in the study was uneventful. He progressed through the baseline and first 3-months post-randomization visits without any problems. At his 3-month follow up visit, he was noted to have a new anemia, for which he was referred to his primary care doctor. Work-up for anemia a month later revealed abnormal LFTs, with a cholestatic pattern: alkaline phosphate (ALP) level was 11 times the upper limit of the local laboratory reference range. The LFT’s were repeated, and following confirmation, his study drugs were stopped and the patient was admitted to the hospital for work-up of cholestatic liver injury. A liver biopsy showed mild portal inflammation that was consistent with an adverse drug reaction. LFTs began to improve in the weeks after stopping NAM (Table). One month after stopping NAM, the patient developed persistent fatigue, recurrent Bell’s palsy, and balance and vision impairment. The LFTs increased again. At the primary care visit, his doctor noted a new palmar rash. Rapid plasma reagin (RPR) tested positive. The patient was diagnosed with secondary syphilis and intravenous (IV) penicillin was prescribed. The patient’s LFTs improved rapidly with IV penicillin (Table), confirming the diagnosis of syphilitic hepatitis as cause of cholestatic liver injury. Discussion We observed an initial improvement in LFTs when the patient stopped taking NAM, which raised the question whether NAM could have caused the cholestatic liver injury in the patient. Prior to the diagnosis of syphilitic hepatitis in this patient, the study team re-examined the literature and reconsidered potential risks to participants. Once secondary syphilis emerged as the unifying cause for the patient’s constellation of symptoms, the study team again reconsidered risks to participants and determined that these were unchanged. The study proceeded as originally planned, and to date there have been no additional incidences of liver injury in the study.