Presenting Author:
Tyler Wanke, MB.A.
Principal Investigator:
Bradley Merk, M.D.
Department:
Orthopaedic Surgery
Keywords:
Orthopaedic Surgery, Trauma, Medical Devices, Depth Gauge, Cleanliness and Sterility, Infection Control, SSI, Surgical S... [Read full text]
Location:
Ryan Family Atrium, Robert H. Lurie Medical Research Center
C71 - Clinical
A Study of Orthopaedic Depth Gauge Contamination Following Routine Cleaning
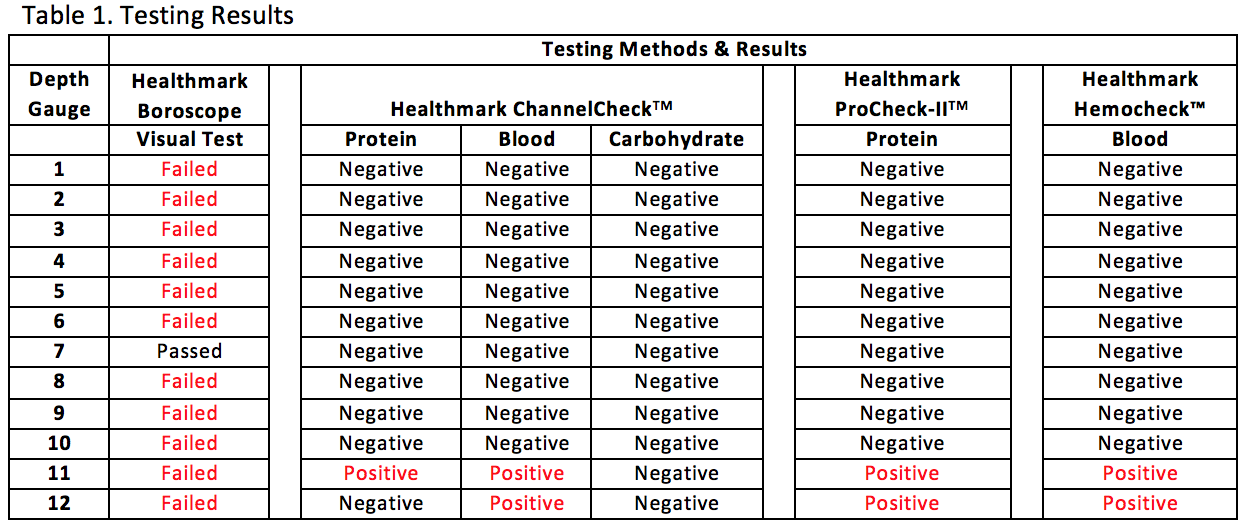
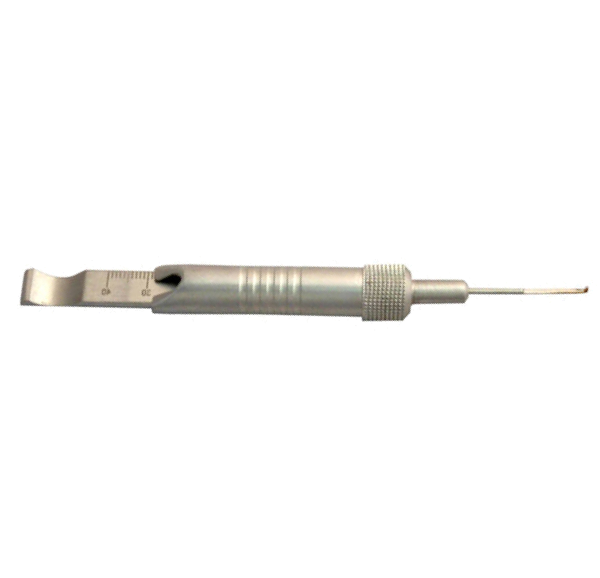
Purpose: Surgical site infections (SSIs) are a serious issue in orthopaedic trauma surgery. One known etiology behind hospital acquired SSIs is the use of contaminated reusable medical devices, particularly those with designs that make effective cleaning difficult, because proper cleaning is essential for effective sterilization. Specifically, devices with narrow, rigid, cannulae or bores and multiple components can retain debris and bioburden within the bore or small spaces of the device. Most of these features exist in orthopaedic depth gauges, which are cannulated tools with multiple components. These are routinely used in trauma surgery to measure the depth of pilot holes for screw length selection during fixation, and, therefore, are regularly exposed to blood, bone, and tissue. The purpose of this study was to measure the cleanliness of orthopaedic depth gauges after standard cleaning and reprocessing. Method: In this experiment, standard visual and chemical tests of device cleanliness were conducted on a sample (n=12) of randomly selected orthopaedic depth gauges at a highly ranked Level I trauma center. All depth gauges were collected on the same operating day in July 2016 after undergoing the center’s standard cleaning processes. The devices were visually inspected for soils, which could include rust, blood, bone, tissue, or other debris. Devices were inspected with the naked eye and with a lighted, flexible 3.3 mm borescope (Healthmark Industries Company, Fraser, MI) which was used to visualize inside the device lumens. The devices were also tested for protein residue (ProCheck-IITM Detection; Healthmark, Fraser, MI; Cat # PT-202) and hemoglobin (Hemocheck™; Healthmark, Fraser, MI; Cat # HC-101), as well as with a combined test for carbohydrate, protein, and hemoglobin (ChannelCheckTM; Healthmark, Fraser, MI; Cat # UCC-001P, Lot+ 65004). Results: Of the devices that were tested, 91.7% (11 devices) failed visual inspection by flexible boroscope, meaning visual evidence of soils – which could be rust, bone, blood, or other contaminant – was evident within the device. Notably, the small size of the lumen made it impossible to visualize the small end of the device lumen, which could have retained additional debris. Furthermore, 16.7% (2 devices) failed chemical tests for hemoglobin and those same two devices tested positive for protein, indicating that at least 0.1 g of hemoglobin and 1 g of protein remained after cleaning (Table 1). Conclusion: Ultimately, the majority of the devices harbored soil. Of greater concern, 16.7% tested positive for a previous patient’s blood residue. These results suggest issues with the design of orthopedic depth gauges, whose narrow lumens create a space that is challenging to clean properly. Thus, new methods to decrease the risk of the orthopedic depth gauge should be considered, including more effective cleaning methods or using a single-use, disposable device.