Presenting Author:
Phillip Lewis, B.S.
Principal Investigator:
Ramille Shah, Ph.D.
Department:
Surgery
Keywords:
Liver, Tissue Engineering, 3D-Printing, Regenerative Medicine, Scaffold
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B174 - Basic Science
3D-Printed gelatin scaffold geometry modulates hepatocyte function and gene expression
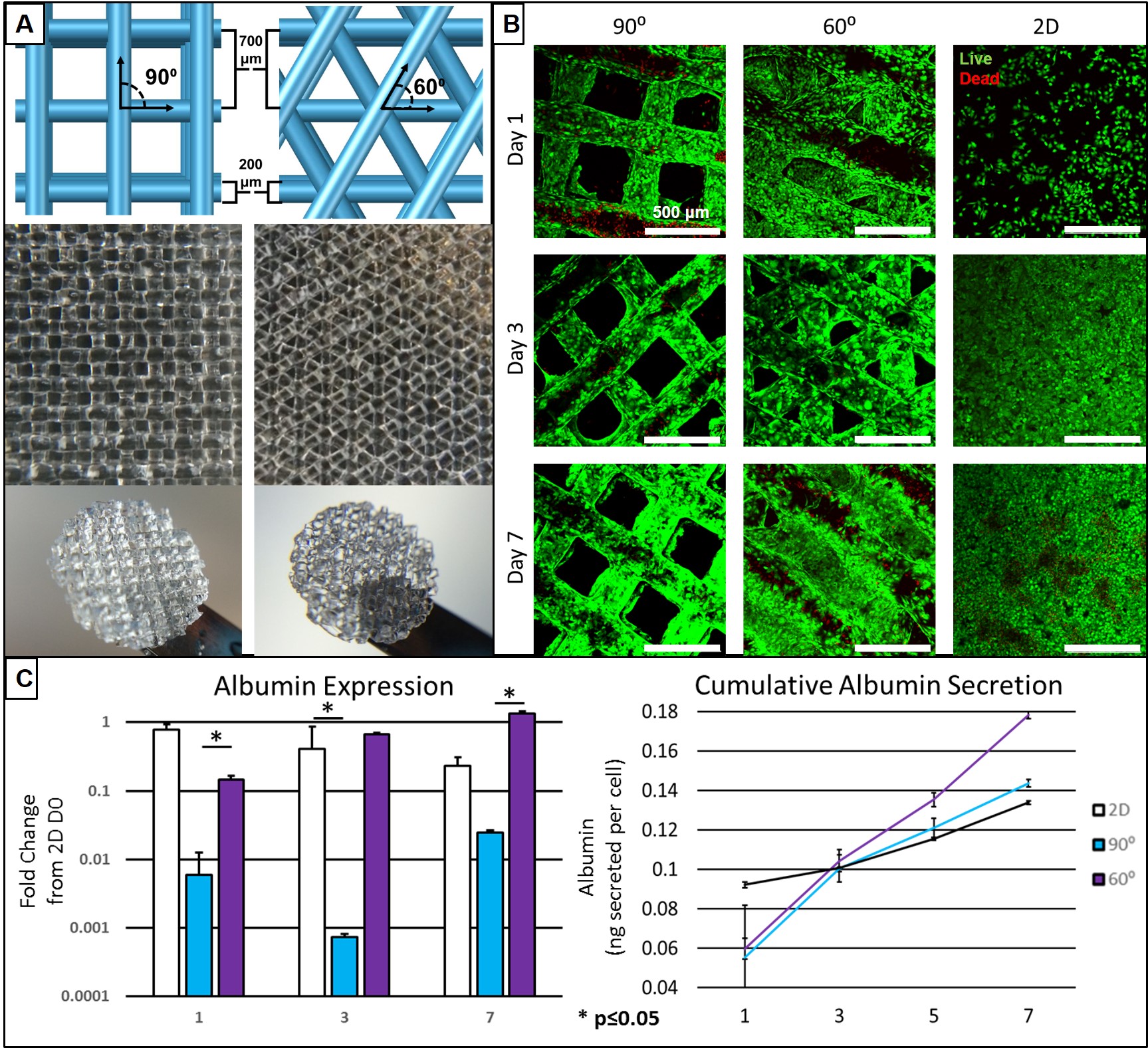
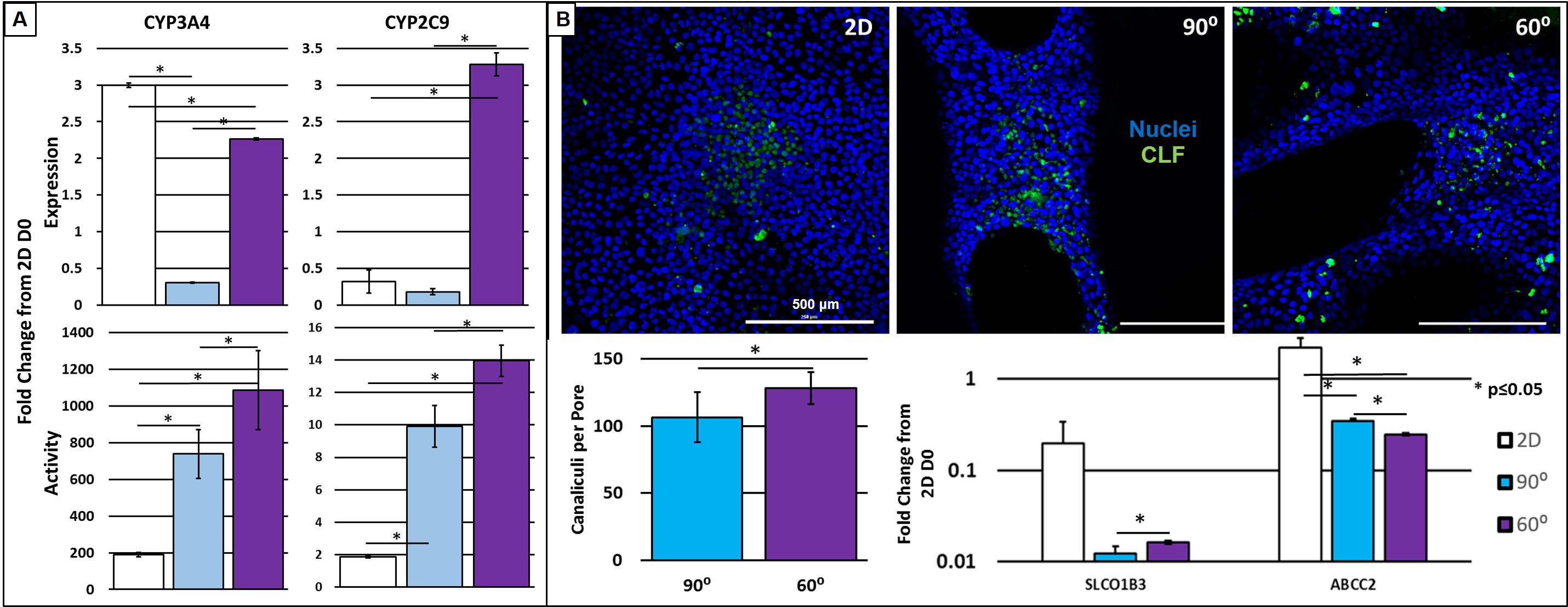
Currently the only viable treatment for end stage liver disease is orthotropic liver transplantation. Transplantation is unfortunately severely limited by the deficit of available donor organs. Tissue engineering (TE) holds promise to mitigate the transplant shortage by providing transplantable tissues and organs, however traditional TE methods have yet to produce significant clinical results. Three dimensional (3D) printing is a new method amenable to the fabrication of TE organs of a repetitive microstructure such as the liver. The creation of uniform and geometrically repetitive tissue scaffolds can allow for the control over cellular aggregation and nutrient diffusion. However, the effect of differing geometries, while controlling for pore size, has yet to be investigated in the context of hepatocyte function. In this study, we show the ability to precisely control pore geometry of 3D-printed gelatin scaffolds (Figure 1A). When seeded on 3D-printed scaffolds of two different geometries (60⁰ and with 90⁰ strut orientations) an undifferentiated hepatocyte cell line (HUH7) demonstrated high viability (Figure 1B). 2D coatings of gelatin were used as a control. Evaluation of albumin secretion and gene expression indicate superior hepatocyte-function in 60⁰ geometries (Figure 1C). However, 2D controls began to demonstrate a disparity between gene expression and assayed function of cytochrome P450 enzymes (Figure 2A). We hypothesized that this was due to the structure of in vivo hepatocytes, and that a 3D environment is necessary for normal hepatocyte polarization and membrane trafficking. To test this, we cultured hepatocytes in the presence of a fluorescent bile salt, cholyl-lysyl-fluorescein (CLF), which localizes to bile canaliculi (Figure 2B). Using confocal microscopy and image analysis, we observed a significantly higher amount of canaliculus formation in the pores of more tortuous 60⁰ geometries. The increased expression of importer proteins (SLCO1B3) and canalicular exporter proteins (ABCC2) in 2D, and a lack of additional canalicular spaces, is additional evidence for the discrepancy between expression and function, and is likely a manifestation of the sensitivity of HUH7 cells and hepatocytes in general to 3D culture. We have demonstrated that 3D spatial patterning of a relatively bland material such as gelatin can influence the function of hepatocytes seeded within scaffold pores by varying a simple feature such as scaffold geometry. Functional tests demonstrated here indicate superiority of 60⁰ scaffolds in certain respects, however, a future 3D-printed liver may incorporate a variety of geometries each designed to encourage a particular hepatocyte function. We foresee a harmony between biology and 3D printed structure, wherein larger organ features such as ducts or blood vessels can be directly manufactured, while 3D placement of multiple cells and biomaterials can guide the formation of smaller structures.