Presenting Author:
Justin Ideozu, Ph.D.
Principal Investigator:
Hara Levy, M.D.
Department:
Pediatrics
Keywords:
ABCC1, cystic fibrosis, DNA methylation, polymorphism
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B148 - Basic Science Women's Health Research
Increased expression of plasma-induced ABCC1 mRNA in cystic fibrosis
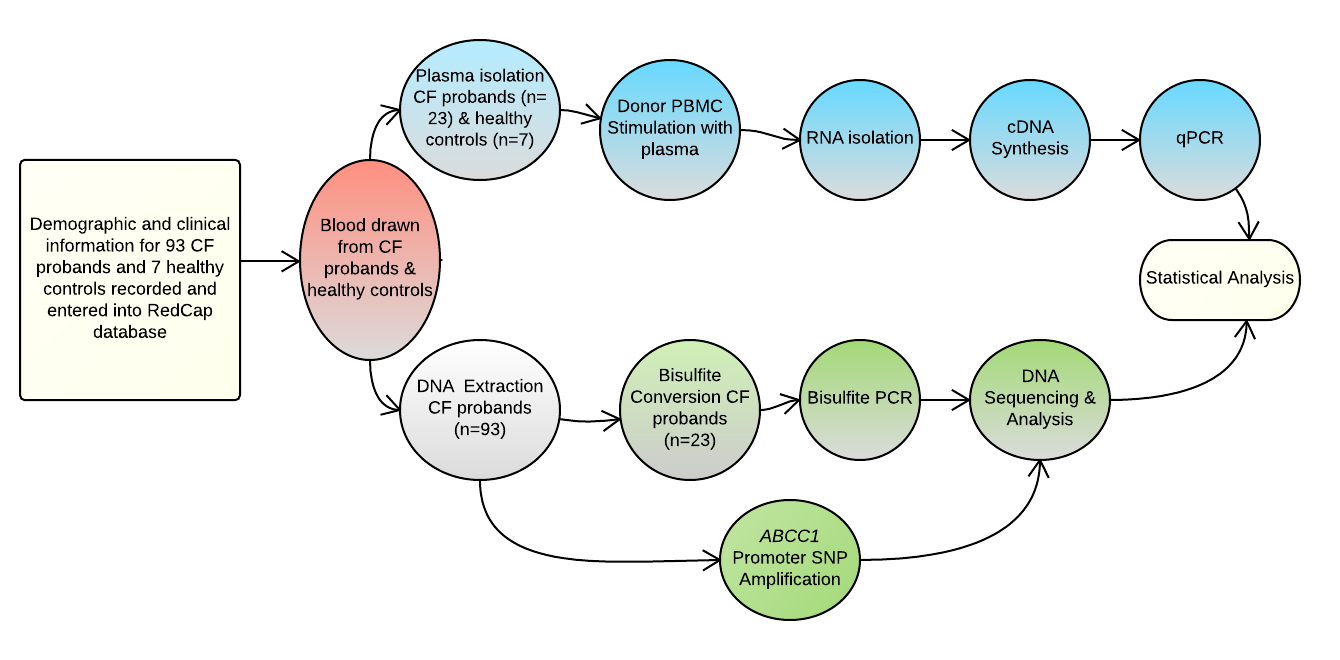
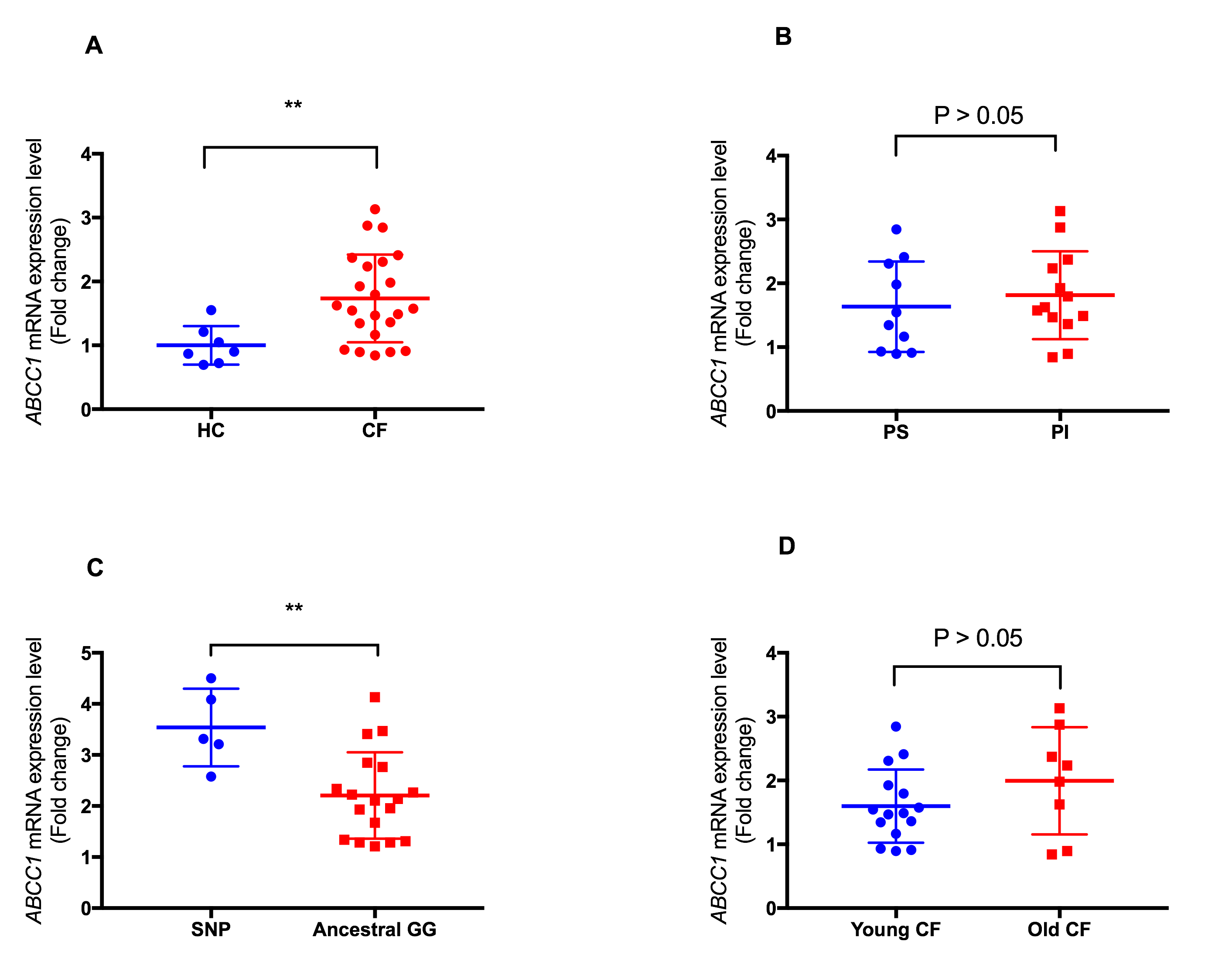
Introduction: The ABCC1 gene has close structural and functional association with CFTR gene, with increased expression thought to play a role in disease manifestations in cystic fibrosis (CF). With up-regulation of ABCC1 resulting in improved lung function in CF patients following treatment with azithromycin, regulatory factors that interact with ABCC1 expression are potential candidates for elucidating how ABCC1 modulates lung disease severity in CF. This study was aimed at analyzing the functional ABCC1 promoter SNP (rs504348), mRNA expression levels and methylation status among CF patients with differing CFTR genotypes and disease severity, and correlation with clinical variables. Methods: Demographic and clinical information including sweat chloride levels, age at diagnosis, growth, lung function, respiratory microbiology, pancreatic sufficient status, and CFTR genotypes were recorded and entered into RedCap database. We analyzed 93 CF probands. All participants were genotyped at the rs504348 SNP. ABCC1 plasma-induced mRNA expression level was analyzed in 23 CF probands and 7 healthy controls. The ABCC1 promoter methylation status was then analyzed in the 23 CF probands following bisulfite PCR and sequencing. Results: Pancreatic insufficient CF probands had a significantly higher positive culture for Pseudomonas aeruginosa compared to those with a milder disease (p = 0.02). A significant association was shown between lung disease severity categories and colonization with P. aeruginosa (p = 0.003). Plasma-induced ABCC1 expression level was significantly higher (p = 0.004) in CF patients with rs504348 compared to those with the ancestral GG genotype. Overall, CF probands had a significant higher expression of ABCC1 mRNA compared to healthy controls (p= 0.001). Conclusion: While CFTR is unique among the ABC subfamily C members of transporters due to its intrinsic ability to conduct chloride ion at a much faster rate, it shares the closest homology with ABCC1. The higher mRNA levels of ABCC1 in CF patients compared to healthy individuals may be due to increased activity of the ABCC1 gene in CF subjects compensating for a diminished CFTR. Also, our study suggests promoter methylation does not seem to modulate ABCC1 expression. Future work is aimed at analyzing other transcriptional regulatory mechanisms.