Presenting Author:
Wenjun Kou, Ph.D.
Principal Investigator:
John Pandolfino, M.D.
Department:
Medicine
Keywords:
transition zone, muscle contraction, esophageal mucosa, esophageal emptying, esophageal peristalsis, esophageal-gastric model
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B45 - Basic Science
Understand esophageal physiology and pathophysiology from fully-coupled fully-resolved simulations
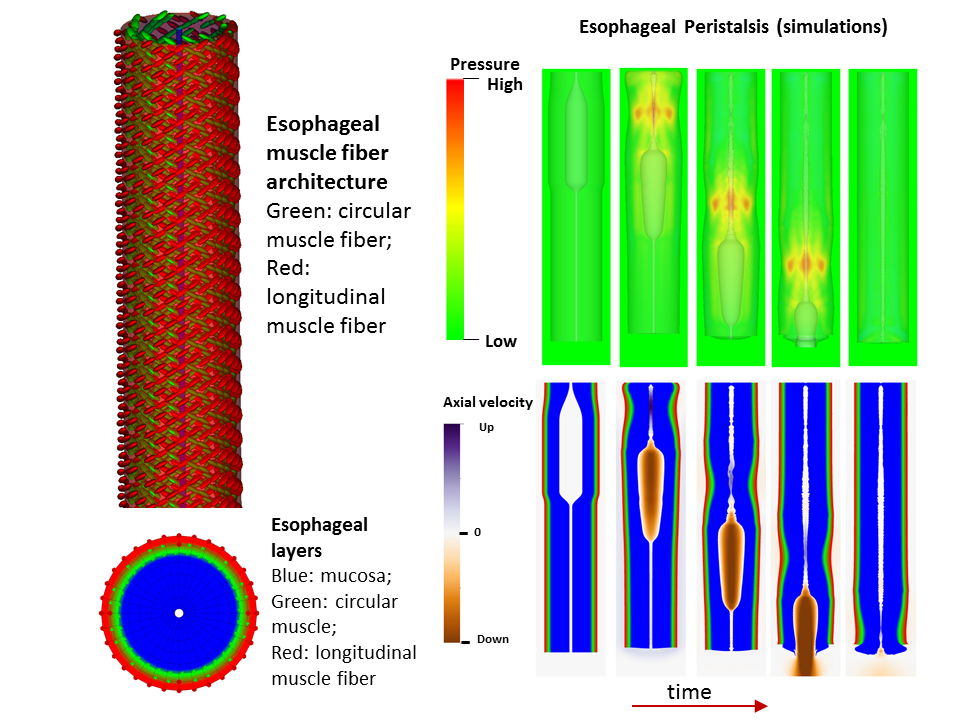
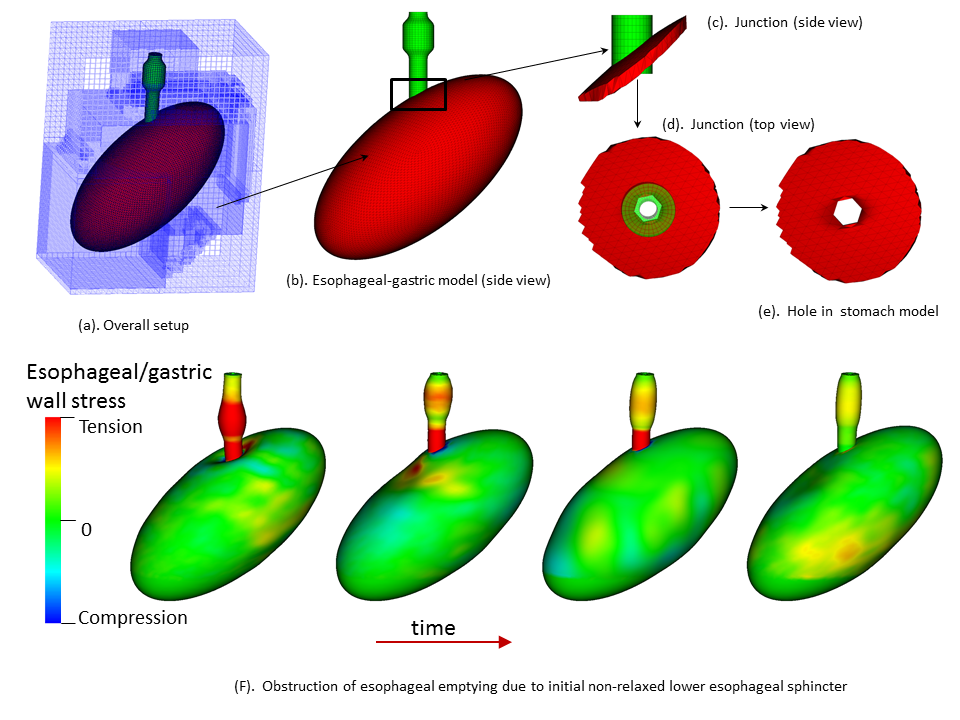
Background: An essential function of the esophagus is to transport ingested food from the pharynx to the stomach, coordinated by neurally-controlled muscle activation, a process called esophageal bolus transit. Successful bolus transit requires a complicated but delicate interaction among (at least) four elements: multi-layered esophageal wall, neurally-controlled muscle contraction/relaxation, gastric wall, and food bolus. Abnormality of any element could potentially promote or cause esophageal dysfunction, such as dysphagia or reflux. Hence, investigating the contribution of each element in the context of complex biophysical interactions is important to advance the understanding of esophageal physiology and pathophysiology, and to provide guidance on related clinical diagnoses and treatments. A challenge for this approach lies in the necessity of resolving the dynamic interactions among the four elements during the coordinated swallowing process. Method: A cross-disciplinary team from the Mechanical Engineering department in McCormick and Gastroenterology & Hepatology in the Department of Medicine, Feinberg, developed a fully coupled fully resolved esophageal transport model. The model is the first of its kind that integrated the above four elements into a single fully resolved simulation. In particular, the model captured multiple layers of the esophageal wall, including the mucosa and muscularis; multiple time-dependent muscle contraction waves, i.e. longitudinal muscle (LM) shortening and circular muscle (CM) contraction. The model was then used to study the functions of LM and CM activation and their relative coordination; the role of the mucosal and the impact of varying its stiffness; the effect of varied muscle fiber architecture and its potential contribution to the mid-esophageal pressure transition zone; and the roles of lower esophageal sphincter (LES) contractility and the gastric wall in facilitating esophageal emptying. Results/Conclusions: A fully-coupled fully-resolved model was developed. The model could simulate case studies that would be difficult, if not impossible, to approach experimentally or with conventional models. Simulation studies have thus far demonstrated that: LM shortening strengthens CM contraction; mucosal compliance is important and a stiffened mucosa can cause obstruction; the pressure transition zone in the mid esophagus may have a myogenic origin related to muscle fiber architecture; a weak LES or stiffened gastric wall may promote formation of lower esophageal diverticuli.