Presenting Author:
Guillermo Ares, M.D.
Principal Investigator:
Catherine Hunter, M.D.
Department:
Pediatrics
Keywords:
Necrotizing enterocolitis, tight junctions, claudin, permeability
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B147 - Basic Science
Necrotizing Enterocolitis Induces Changes in Intestinal Epithelial Tight Junctions
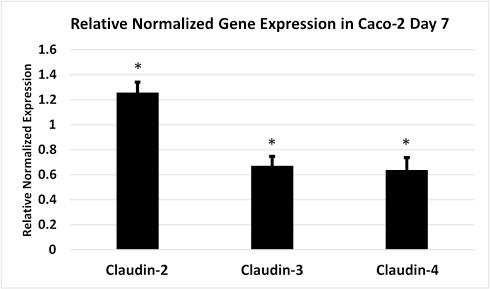
Introduction: Necrotizing enterocolitis (NEC) is a devastating gastrointestinal emergency in neonates, associated with high morbidity. Epithelial tight junction proteins, including claudins, are essential for intestinal barrier function. We hypothesized that pore-forming claudins (type-2) are overexpressed, and strengthening claudins (types-3 & 4) are under-expressed in NEC. Methods: Experimental NEC was induced in Caco-2 cells exposed to lipopolyssacharide and in rat pups subjected to hypoxia and bacteria-containing formula. Pup intestine and cell culture permeability were assayed by FITC. RNA and proteins were extracted for western blot analysis and qPCR for claudins. Sub-cellular fractionation localized claudin proteins. Following IRB approval, human tissue samples from patients with and without NEC were collected and assayed with immunofluorescence. Data were analyzed with Student’s t-test where appropriate. Results: Permeability increased in rat pups and Caco-2 experimental NEC groups (p<0.03). qPCR revealed a 2-fold increase in claudin-2 expression in rats and Caco-2 with NEC (p<0.007). Western blot analysis confirmed increased in claudin-2 protein in experimental NEC (p<0.05). Sub-cellular fractionation localized this increase within the membrane compartment vs. cytosol. Conversely, claudin-3 and 4 had decreased expression in human and experimental NEC by qPCR and Western blot (p<0.005). Sub-cellular fractionation localized the decrease within the membrane compartment. This was further confirmed by immunofluorescence in experimental and human NEC (p<0.005). Conclusion: Experimental NEC demonstrated increased intestinal permeability compared to controls. This physiologic change is associated with increased pore forming claudin-2 and decreased strengthening claudins-3 and 4 in human and experimental NEC. Further research identifying therapeutics targets to modulate tight junction proteins in NEC is warranted.