Presenting Author:
Joseph DiDomenico, B.S.
Principal Investigator:
Orin Bloch, M.D.
Department:
Neurological Surgery
Keywords:
glioblastoma, pd-l1, b7-h1, immunotherapy, progression free survival
Location:
Ryan Family Atrium, Robert H. Lurie Medical Research Center
C50 - Clinical
Peripheral PD-L1 does not predict survival for newly diagnosed glioblastoma
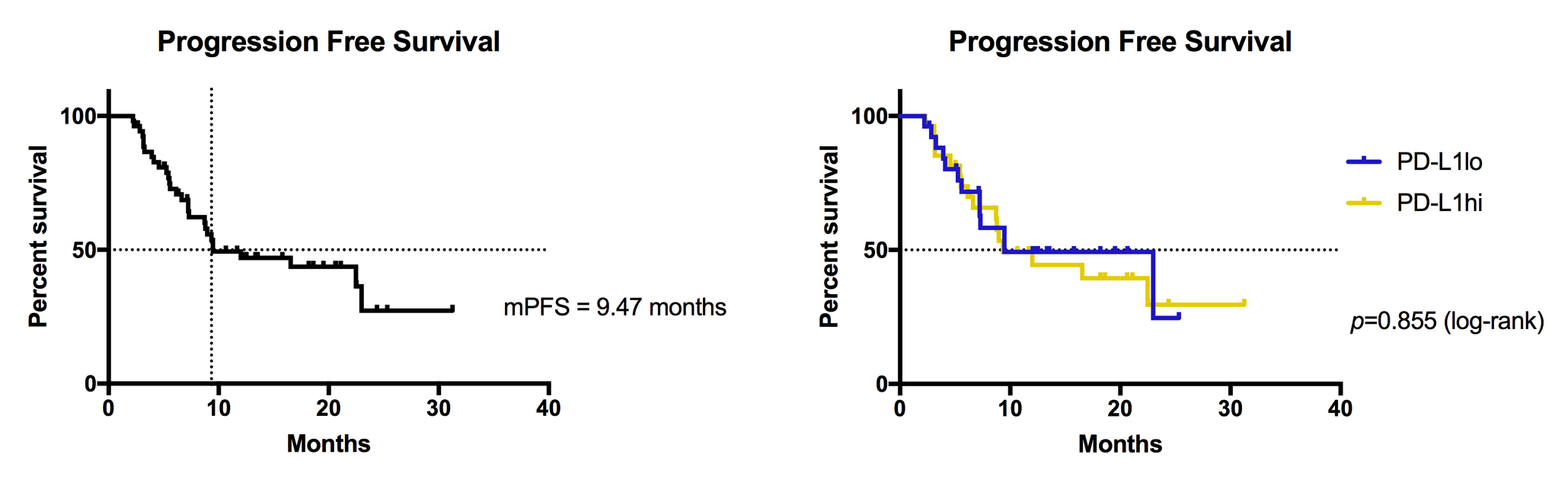
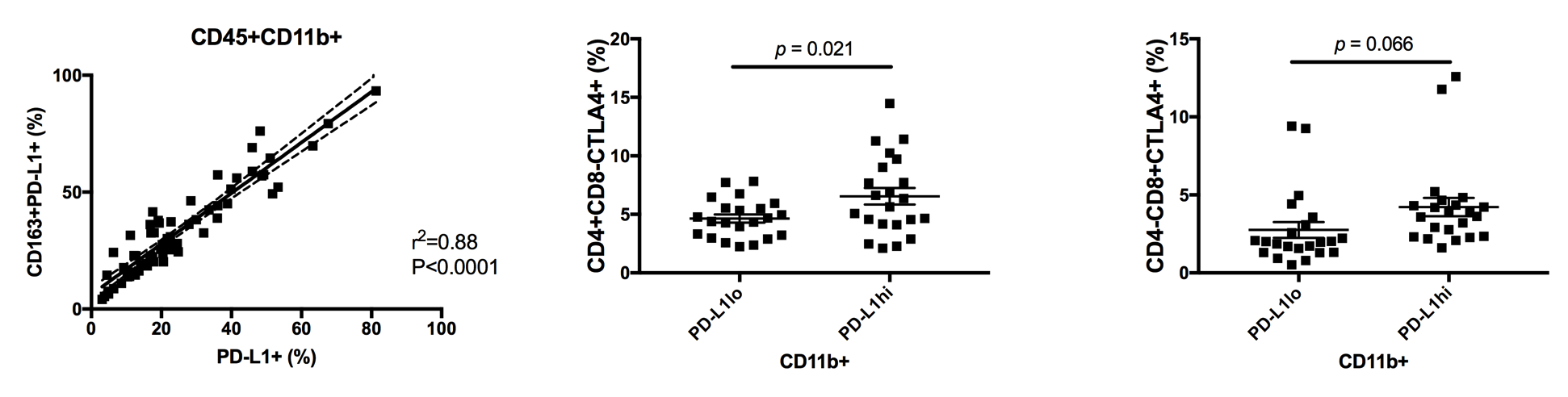
Introduction: Glioblastoma (GBM) induces suppression of the patient immune response through the upregulation of programmed death-ligand 1 (PD-L1). Intratumorally, PD-L1 is most expressed on tumor cells and associated macrophages, and systemically, it is induced on peripheral monocytes. Disrupted interaction of PD-L1 with its receptor, PD-1, has received considerable attention as an immunotherapeutic measure, supporting the anti-tumor activity of directed T lymphocytes. Here, we investigate the clinical significance of peripheral PD-L1 expression for glioblastoma patients. Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of patients with newly diagnosed GBM (n=53). Monocytic (CD45+CD11b+CD163+/-) PD-L1 positivity and T cell fractions (CD3+CD4+/-CD8+/-CD25+/-FoxP3+/-) were quantified by flow cytometry, and T cells were assessed for exhaustive marker CTLA4. Patients were divided into PD-L1hi and PD-L1lo subsets. Patients were followed through their treatment course, with all receiving maximal resection followed by standard chemoradiation. Radiologic progression and survival were recorded. Results: Mean follow-up time was 13.67 months. Overall, the cohort had a median progression free survival (PFS) of 9.47 months. PFS for PD-L1hi and PD-L1lo subsets was 9.34 and 9.47 months, respectively (HR = 1.12; 95% CI, 0.54 to 2.32; P= 0.855 by log-rank test). Total peripheral monocyte (CD45+CD11b+) and M2 macrophage (CD45+CD11b+CD163+) PD-L1 expressions positively correlated (R2=0.88, P<0.0001). Patients in the PD-L1hi group had greater CTLA4 expression on CD4+CD8- (P=0.02) and CD4-CD8+ T cells (P=0.07) compared to those in the PD-L1lo group. Conclusions: The role of PD-L1 in glioma-associated immunosuppression has been well established. This is reflected in our data, with peripheral monocyte PD-L1 expression strongly correlating with that of the M2 macrophage immunosuppressive subset, and greater PD-L1 expression associating with the upregulation of T cell exhaustive markers. Several clinical trials are currently underway to assess the clinical effect of blocking PD-L1 function. We demonstrate, though, that peripheral PD-L1 expression does not impact time to progression. This finding suggests that PD-L1 alone cannot predict survival. However, PD-L1 blockade may best serve as an adjunct to immunotherapeutic interventions, such as vaccine therapy, that demonstrate diminished efficacy with peripheral upregulation of this ligand.