Presenting Author:
Shin Yoo, Ph.D.
Principal Investigator:
Rishi Arora, M.D.
Department:
Medicine
Keywords:
Parasympathetic nerve, electrical remodeling, G proteins, Atrial fibrillation
Location:
Third Floor, Feinberg Pavilion, Northwestern Memorial Hospital
B38 - Basic Science
Inhibition of parasympathetic signaling by Gαi/o peptides prevents atrial fibrillation
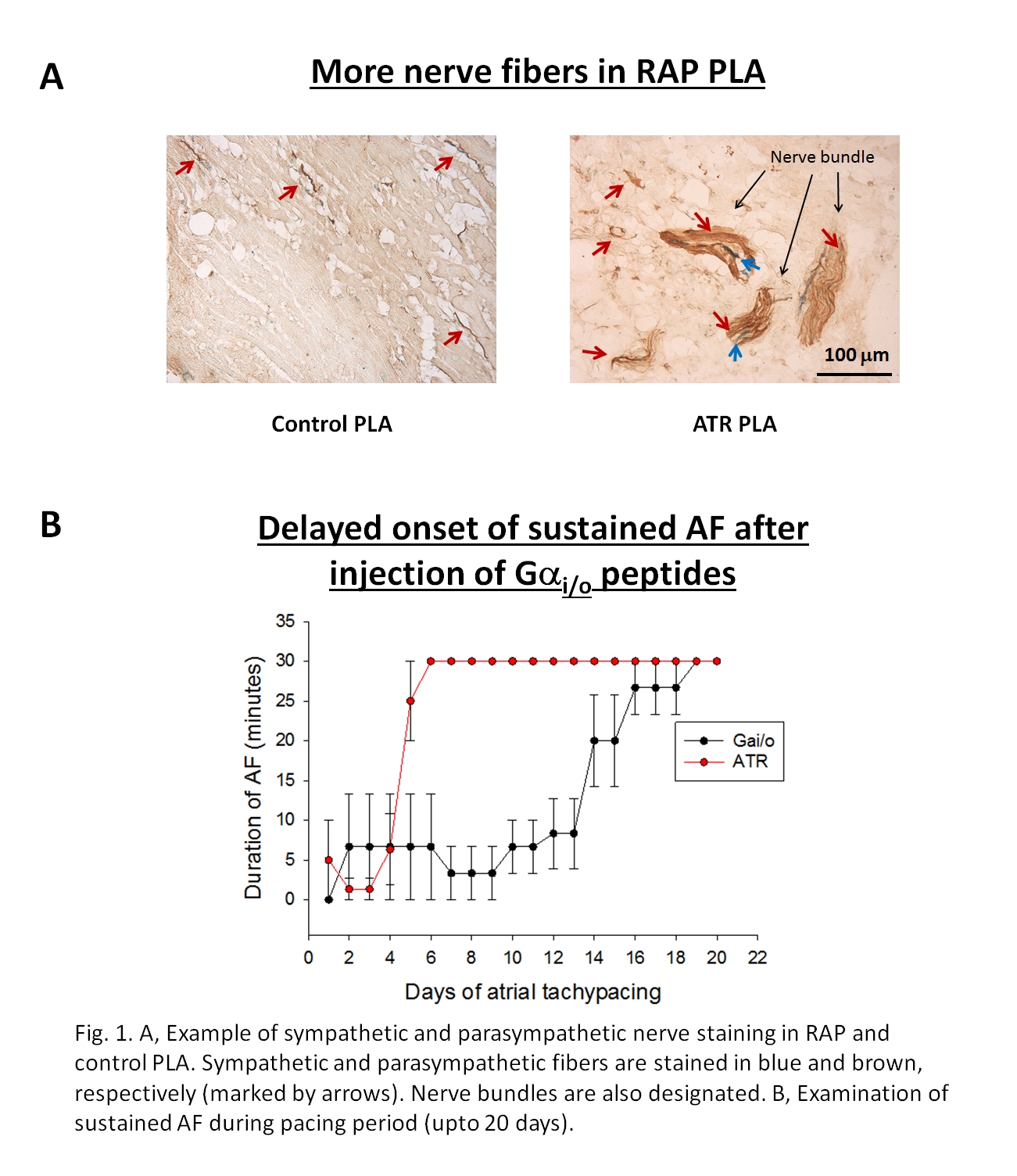
Introduction: Since the autonomic nervous system (ANS) is thought to contribute to the genesis of atrial fibrillation (AF), we hypothesized that parasympathetic (P) hyper‐innervation is an important contributor to refractory period shortening (electrical remodeling) in AF. We therefore: a) assessed P innervation in a canine model of rapid atrial pacing (RAP) induced AF and b) performed targeted inhibition of P signaling in RAP dogs by using minigenes expressing novel C‐terminal Gi/o peptides. Methods: P innervation was assessed in the left atrium of 5 dogs subjected to three weeks of RAP (600 beats/min for 3 weeks) and 5 control dogs by immunostaining for acetylcholinesterase. 4 other dogs were subjected: a) atrial injection of minigene expressing Gαi2+Gαo1 inhibitory peptides (N = 2) or b) scrambled gene or sham injection (N = 2), followed by 3 weeks of RAP. All RAP dogs were followed for development of AF. Results: RAP dogs had a significantly larger number of P nerves than controls (RAP, 8.33 ± 0.34 vs. control, 7.62 ± 0.99 (density of individual P fibers in atrial myocardium), p < 0.05; fig. 1A). RAP dogs that did not receive gene or underwent scrambled/sham gene injection developed AF after 6 ± 1 days of pacing (fig. 1B). In contrast, in dogs receiving Gαi/o peptides the onset of AF was delayed by more than a week (p < 0.05) (fig. 1B). Conclusion: Persistent AF leads to aggressive P nerve growth in the atrium, with these nerves appearing to significantly contribute to electrical remodeling in AF. Targeted inhibition of P signaling by Gαi/o peptides significantly attenuates electrical remodeling AF, indicating that such an approach may have therapeutic potential in AF.