Presenting Author:
Joseph Paonessa, M.D.
Principal Investigator:
Richard Wunderink, M.D.
Department:
Medicine
Keywords:
MRSA, Methicillin-Resistant Staphylococcus aureus, pneumonia, mechanical ventilation, antibiotic stewardship
Location:
Ryan Family Atrium, Robert H. Lurie Medical Research Center
C49 - Clinical
Use of Automated PCR to Detect MRSA in Bronchoalveolar Lavage:
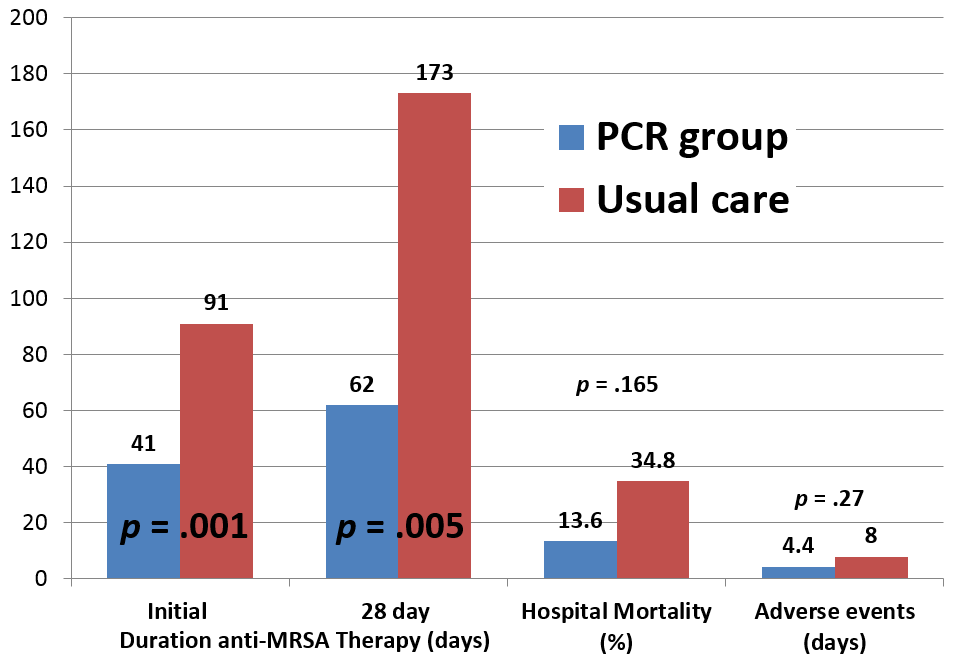
Rationale. The frequency of MRSA pneumonia in ventilated patients is sufficiently high in most institutions that empirical linezolid or vancomycin is recommended until culture results are available. Even short courses of anti-MRSA agents may alter host flora and have toxicity, particularly vancomycin. A rapid diagnostic test using automated polymerase chain reaction (A-PCR) assay has been shown in several laboratory studies to detect both MSSA and MRSA in bronchoalveolar lavage (BAL) with a high negative predictive value. Our aim was to prospectively determine if anti-MRSA agent administration could be safely decreased by early use of A-PCR to detect MRSA in BAL fluid in the medical intensive care unit (MICU). Methods. We prospectively enrolled mechanically ventilated adults with residual BAL samples while being empirically treated with anti-MRSA antibiotics for suspected pneumonia in the MICU of a single large academic center from May 2016 to January 2017. Exclusion criteria included: neutropenic fever, chronic airway infection, suspicion for extra-pulmonary MRSA infection, patient/surrogate refusal, physician refusal to narrow antibiotics based on A-PCR, or >48 hours of anti-MRSA therapy prior to enrollment. Patients were randomized to either receive A-PCR testing or to continue with usual care. Given nature of study, there was no blinding of patient/surrogate, physician, or study team. For the A-PCR group, BAL samples were tested using the MRSA/SA SSTI Assay on the Cepheid Xpert® platform. Results were compared to quantitative BAL cultures, with positive test defined as bacterial growth >103 cfu/ml. Primary outcome was duration of anti-MRSA agent administration. Secondary outcomes included nephrotoxicity, thrombocytopenia, hospital-acquired infections, 28 day in-hospital mortality, and length of stay. Results. A total of 45 patients were randomized (22 A-PCR, 23 control) without any crossover between groups. Initial anti-MRSA agent duration significantly decreased in the A-PCR group (41 vs. 91 hours, p = 0.001) as well 28 day total anti-MRSA agent duration (62 vs. 173 hours, p = 0.005) when compared to usual care group. A-PCR was positive in one patient, with no false negatives compared to culture. A-PCR patients had trends to fewer days of pre-specified adverse events (4.4 vs. 8.0 days, p = 0.27) and decreased unadjusted in-hospital mortality (13.6% vs. 34.8%, p = 0.16). Conclusion. Anti-MRSA treatment can be safely discontinued significantly earlier based on BAL A-PCR results in mechanically ventilated patients with suspected pneumonia. Less anti-MRSA antibiotic use based on A-PCR results was also associated with trends in lower adverse events and in-hospital mortality.