Presenting Author:
Brannan Griffin, M.D.
Principal Investigator:
Luis Blanco Jr., M.D.
Department:
Pathology
Keywords:
HER2 FISH testing, equivocal HER2 results, alternate reference probe D17S122, breast carcinoma
Location:
Ryan Family Atrium, Robert H. Lurie Medical Research Center
C77 - Clinical Women's Health Research
Alternate Probe Establishing HER2 Status In Double Equivocal Breast Carcinomas
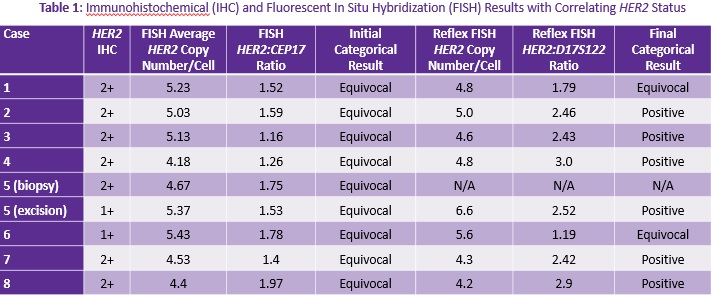
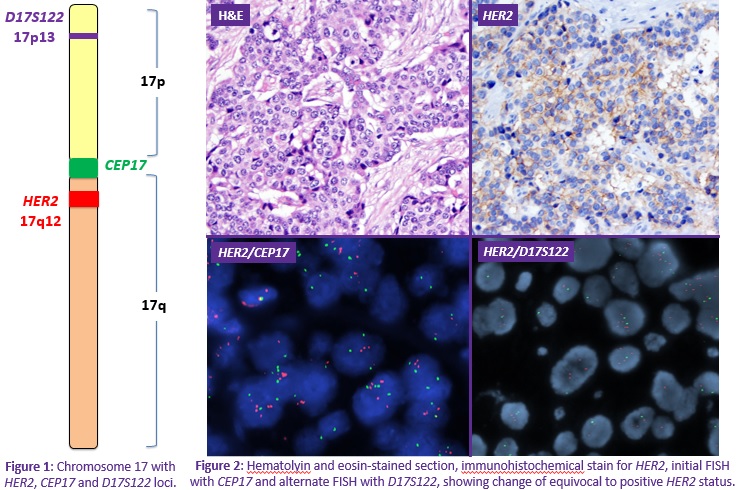
Background: HER2 is an established prognostic factor and vital therapeutic target in the management of breast carcinomas. In 2013, the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for HER2 evaluation were updated. Reflex testing using an alternate method is recommended on cases with equivocal results via immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). However, therapeutic dilemmas arise when both tests are equivocal. The standard chromosome 17 centromere reference probe (CEP17) is in close proximity to the HER2 locus and may be co-amplified leading to equivocal results. Alternate chromosome 17 reference probes may aid in establishing the true HER2 status. However, the current guidelines do not have a specific recommendation for the use of these probes and for which probe to use. Objective: In this study, our goal was to assess the utility of an alternate reference probe, specifically D17S122, in reflex FISH testing to clarify the true HER2 status in cases with equivocal (or discordant) results by both IHC and FISH studies. Methods: Fifteen patients with double equivocal invasive breast carcinomas (or discordant results) diagnosed in 2015-2016 were reviewed. Double equivocal results were defined as an initial HER2 IHC score 2+, and subsequent FISH with HER2 to CEP17 ratio <2.0 and with an average 4 to <6 HER2 signals per cell. Reflex FISH testing was performed with alternate reference probe D17S122 and classified accordingly for eight cases. Results: The results are summarized in Table 1. • Six of eight cases (75%) had a definitive HER2 status with D17S122. Six cases were ultimately classified as positive (75%), while two remained equivocal (25%). • The HER2 status of our two cases with initially discordant results was also resolved by alternate probe testing. The first case was a double equivocal on biopsy with subsequent IHC negative/FISH equivocal results on excision that was ultimately classified as HER2 positive with the alternate probe. The second was an IHC negative/FISH equivocal case that remained equivocal with reflex alternate probe testing. Conclusions: 1. The use of alternate reference probe D17S122 yielded definite results in the majority of cases (75%). 2. More than half of cases were ultimately classified as positive on reflex testing and were therefore treated appropriately with anti-HER2 therapy. 3. Reflex testing with alternate reference probes may establish true HER2 status and ultimately direct proper therapeutic management. For optimal patient care, pathologists should utilize necessary alternate testing to resolve HER2 double equivocal cases. Additional studies are needed to further evaluate D17S122 and other available reference probes to determine which probe(s) are optimal for reflex testing. Finally, the ASCO/CAP guidelines may need to be updated to reflect specific recommendations for the use of these probes in double equivocal cases.